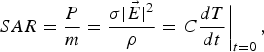I. INTRODUCTION
Recent advances in millimeter-wave technologies have triggered an exponential interest to wireless applications at millimeter waves. Antennas and devices operating in this band have a reduced size compared to their counterparts in the lower part of the microwave spectrum. Furthermore, very high data rates (up to 4 Gb/s) can be reached for short- or long-range communications. The radio coverage strongly depends on frequency and is essentially determined by the water vapor and molecular oxygen-induced atmospheric absorptions. For instance, shifting the frequency from 70/80 to 60 GHz decreases the operating range from 3 km to 400 m [Reference Wells1]. This decrease is related to the 16 dB/km peak of oxygen-induced absorption around 60 GHz [Reference Straiton2, Reference Rosenkranz3] that makes this frequency range extremely attractive for secured local communications, particularly in indoor environments, guaranteeing low interference with other wireless services and devices, as well as with adjacent network cells. At the same time, because of this resonance absorption, human body has never been exposed to 57–64 GHz radiations in the natural environmental conditions.
60-GHz broadband short-range communications for wireless personal area networks have been promoted by the WirelessHD Interest Group and WiGig alliance. The current target market applications are mainly restricted to indoor wireless high-definition multimedia devices [Reference Daniels, Murdock, Rappaport and Heath4]. Integrated 60-GHz front-ends are expected to be commercialized by 2014 on lap tops. Moreover, recent progress in miniaturization and low-cost devices has triggered research activities aiming at developing future millimeter-wave body area networks (BAN). Recently, several world-leading research groups have focused on the characterization of the body channel, development of on-body antennas, and integration with already existing devices, e.g. [Reference Hall5].
Before being introduced on the market, millimeter-wave systems should comply with local regulations that are usually based on the ICNIRP and/or IEEE exposure limits. For far-field exposures, the power density (PD) averaged over 20 cm2 is limited to 1 mW/cm2 (general public) and to 5 mW/cm2 (workers) in the 60-GHz band [6, 7]. To respect these limits and due to technological limitations, the typical power radiated by the radio front-ends is below 10 dBm. However, power densities up to 20 mW/cm2 (general public) and 100 mW/cm2 (workers) are permitted for local exposure scenarios, i.e. for PD averaged over 1 cm2 [6]. Exposures under these conditions have had a limited practical interest so far, but should now be studied in detail due to the expected development of body-centric communication systems [Reference Hall5, Reference Xiao, Zhou and Zhang8, Reference Smulders9]. In such systems, the antennas might be placed directly on the body inducing localized exposures of the superficial body layers.
In this context, it is fundamental to analyze millimeter-wave/human body interactions from electromagnetic (EM) and thermodynamic viewpoints, as well as the potential biological consequences and their power thresholds.
The main purpose of this paper is to make an overview of the most recent results related to the different aspects of millimeter-wave interactions with the human body.
II. INTERACTION OF THE MILLIMETER WAVES WITH THE HUMAN BODY
A) Primary biological targets for 60-GHz radiations
The primary biological targets of 60-GHz radiations are the skin and eyes. Exposure of the eyes leads to the absorption of the EM energy by the cornea characterized by a free water content of 75% and a thickness of 0.5 mm. Ocular lesions have been found after high-intensity exposure of an eye (3 W/cm2, 6 min) [Reference Kojima10]. However, studies performed at 60 GHz (10 mW/cm2, 8 h) demonstrated no detectable physiological modifications [Reference Kues, D'Anna, Osiander, Green and Monahan11], indicating that millimeter waves act on the cornea in a dose-dependent manner.
Hereafter we will essentially consider the interactions with the skin as it covers 95% of the human body surface. From the EM viewpoint, the skin can be considered as an anisotropic multilayer dispersive structure made of three different layers, namely, epidermis, dermis, and subcutaneous fat layer (Fig. 1). The skin also contains capillaries and nerve endings. It is mainly composed of 65.3% of free water, 24.6% of proteins, and 9.4% of lipids [Reference Duck12].

Fig. 1. Schematic representation of the skin structure.
B) Dielectric properties
Knowledge of the dielectric properties of the skin is essential for the determination of the reflection from, transmission through, and absorption in the body, as well as for EM modeling. In contrast to frequencies below 20 GHz, existing data on the permittivity of tissues in the millimeter-wave band are very limited due to some technical difficulties. In the 10–100 GHz range, the dispersive dielectric properties of the skin and biological solutions are primarily related to the rotational dispersion of free water molecules. In particular, high losses are related to the free water relaxation with the peak at 19 GHz at 25°C [Reference Ellison13].
The results of skin permittivity measurements reported in the literature so far have demonstrated strong correlation with the measurement technique and skin model (in vivo or in vitro, skin temperature, location on the body and thicknesses of different skin layers). Gabriel et al. [Reference Gabriel, Lau and Gabriel14] reported extrapolated complex permittivity of human skin up to 110 GHz based on measurements performed below 20 GHz. The results presented by Gandhi and Riazi [Reference Gandhi and Riazi15] at 60 GHz were obtained using a Debye's model based on measurements performed for the rabbit skin at 23 GHz. Alabaster [Reference Alabaster16] measured the complex permittivity of excised human skin samples at millimeter waves using a free-space technique. Hwang et al. [Reference Hwang, Yim, Cho, Cheon and Kwon17] completed in vivo measurement on human skin up to 110 GHz using a coaxial probe. Finally, Alekseev and Ziskin [Reference Alekseev and Ziskin18] carried out reflection measurement with an open-ended waveguide and proposed a homogeneous and multilayer human skin models fitting the experimental data.
The permittivity data available at 60 GHz are summarized in Table 1 for the room and body temperatures. The complex dielectric constant extracted from Gabriel's, Gandhi's, and Hwang's models at 55–65 GHz are presented in Fig. 2. These data are compared with the theoretical values obtained using Maxwell's mixture equation [Reference Zhadobov, Sauleau, Le Dréan, Alekseev and Ziskin19]. Significant discrepancies among the reported data are found for the real (11%) and imaginary (62%) parts (Gabriel's model is used as a reference).
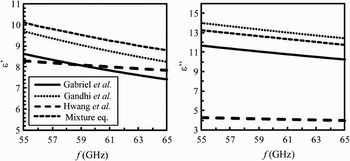
Fig. 2. Complex permittivity of the skin in 55–65 GHz range.
Table 1. Overview of the skin electrical properties at 60 GHz.
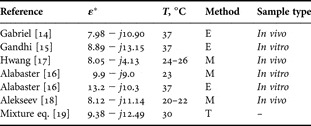
E, extrapolation; M, direct measurement; and T, theoretical value.
C) Dosimetric quantities at millimeter waves
The major dosimetric quantities at millimeter waves are the following:
1) Incident PD: This is the main exposure characteristic adopted by most of the international guidelines and standards in the 10–300 GHz frequency range. The PD is defined as follows:
(1)where P is the incident power, S is the exposed surface area, and
 and
and  are the electric and magnetic field vectors, respectively.
are the electric and magnetic field vectors, respectively.2) Specific absorption rate (SAR): The SAR is a quantitative measure of power absorbed per unit of mass and time. In contrast to the PD, it also takes into account the physical properties of exposed samples:
(2)where m is the tissue mass, σ is its conductivity, and ρ is its mass density. C is the heat capacity, and T is the temperature. It is important to underline that in equation (2) σ combines both electrical and ionic conductivities.
3) Steady-state and/or transient temperature (T), which is particularly important in case of medium- and high-power exposures.
D) Reflection and transmission at the air/skin interface
The skin dielectric properties have a significant influence on the reflection and transmission at the air/skin interface. Their variability should be carefully taken into account for the on-body channel modeling.
For a 60-GHz plane wave illuminating a homogeneous semi-infinite flat skin model, analytically calculated reflection and transmission coefficients are represented in Fig. 3 for the perpendicular polarization (![]() perpendicular to the plane of incidence, TE mode) and parallel polarization (
perpendicular to the plane of incidence, TE mode) and parallel polarization (![]() parallel to the plane of incidence, TM mode). As expected, the reflected power strongly depends on the angle of incidence and polarization. For normal incidence, 30–40% of the incident power is reflected from the skin.
parallel to the plane of incidence, TM mode). As expected, the reflected power strongly depends on the angle of incidence and polarization. For normal incidence, 30–40% of the incident power is reflected from the skin.
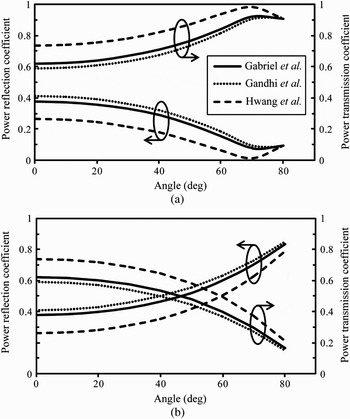
Fig. 3. Power reflection and transmission coefficients at the air/skin interface at 60 GHz for (a) parallel polarization and (b) perpendicular polarization.
E) Absorption in the skin
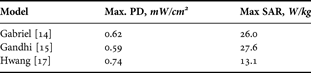
The transmitted power decreases exponentially in the skin as a function of depth. The attenuation of the PD and SAR at 60 GHz are plotted in Fig. 4. The PD and SAR are maximal at the skin surface (Table 2). 40% (for Gabriel and Gandhi models) and 60% (for Hwang model) of the incident power reach dermis; only 0.1% (for Gabriel and Gandhi models) and 10% (for Hwang model) reach the fat layer. These results suggest that only epidermis and dermis should be considered for the EM dosimetry and on-body antenna characterization. This conclusion was confirmed by detailed analysis performed by Alekseev et al. [Reference Alekseev, Radzievsky, Logani and Ziskin20] for multilayer skin models.
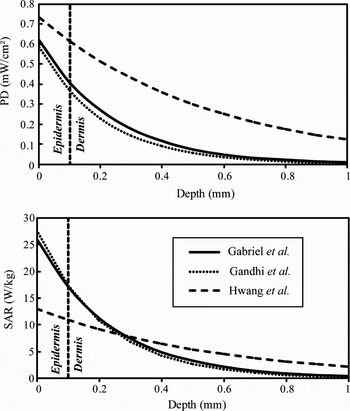
Fig. 4. Attenuation of the PD and SAR in the skin for an incident PD of 1 mW/cm2 at 60 GHz.
Table 2. PD and SAR in the skin for different dielectric models (incident PD = 1 mW/cm2).
F) Influence of clothing
For future body-centric applications, it is particularly important to assess the effect of clothing on the millimeter-wave absorption. The dielectric constant of clothing ɛ c has never been investigated at 60 GHz. Here we assumed ɛ c = 1.25 + j0.024; this corresponds to the complex permittivity of the dry fleece at microwave frequencies [Reference Hertleer, Tronquo, Rogier, Vallozzi and Van Langenhove21]. Since the clothing thickness d c typically ranges between 0 and 1.2 mm, we computed the transmission coefficient at 60 GHz for this range using Gabriel's permittivity model [Reference Gabriel, Lau and Gabriel14]. The results show that the transmission coefficient slightly increases with the clothing thickness (Fig. 5).
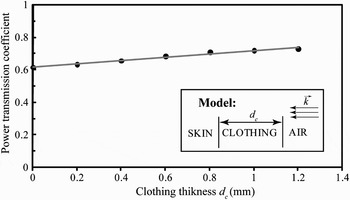
Fig. 5. Comparison of the transmission coefficient with and without clothing at 60 GHz.
Furthermore, to assess the contribution of an air gap d a between the skin and clothing, we computed the power transmission coefficient for a four-layer model (Fig. 6). For a separation ranging from 0 to 2 mm, this coefficient varies from 56 to 72% with a minimum at 1.25 mm. These results demonstrate that, in contrast to the clothing that can enhance the transmission, the presence of an air gap induces a decrease of the power transmitted to the skin.
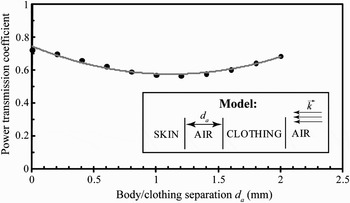
Fig. 6. Power transmission coefficient as a function of the air gap for d c = 1.25 mm at 60 GHz.
G) Millimeter-wave heating
Shallow penetration depth of 60-GHz radiations in the skin (typically 0.5 mm) results in SAR levels that are significantly higher than those obtained at microwaves for identical PD values. This may lead to a significant heating, even for low-power exposures. The steady-state distribution of the relative temperature increments for a PD of 1 and 5 mW/cm2 is represented in Fig. 7. These results correspond to the analytical solution of the 1-D heat transfer equation that takes into account the effect of surface cooling and blood flow [Reference Foster, Kritikos and Schwan22]. It is worthwhile to note that the heating is strongly correlated with the coefficient characterizing the heat transfer from the skin to air. These results demonstrate that heating due to local millimeter-wave exposure affects not only skin, but also subcutaneous tissues including fat and muscles. Therefore, a multilayer model should be used for the accurate assessment of the thermal effects.
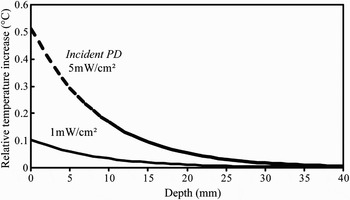
Fig. 7. Temperature increments for a homogeneous skin model exposed to a plane wave at 60 GHz.
The parametric study performed by Kanezaki et al. [Reference Kanezaki, Hirata, Watanabe and Shirai23] demonstrated that the temperature distribution induced by a millimeter-wave exposure strongly depends on the geometrical and thermal properties of the multilayer model. Furthermore, Alekseev and Ziskin [Reference Alekseev and Ziskin24, Reference Alekseev and Ziskin25] demonstrated that heating is related to the blood flow in the skin, i.e. to the environmental temperature and physiological conditions. It was shown that depending on these parameters steady-state temperature increments may vary by a factor of 3.
Finally, it is important to underline that temperature increments induced by the PD below current international exposure limits [6] are much lower than environmental thermal fluctuations.
III. INSTRUMENTATION AND DOSIMETRY FOR THE BIOCOMPATIBILITY STUDIES AT MILLIMETER WAVES
Nowadays, there is an increasing interest of the scientific community to the potential biological effects of millimeter waves [Reference Wells1]. The mechanisms of potential bioelectromagnetic interactions are not fully understood. Progress in this direction may result in new applications, but also in update of current exposure safety standards.
A) Exposure systems
Specific exposure systems have been used to experimentally investigate possible biological impacts of millimeter-wave exposures. Most of these systems can be divided in three main sub-units: (1) signal generation sub-unit; (2) spectrum and power control sub-unit; and (3) exposure chamber containing biological samples (Fig. 8).
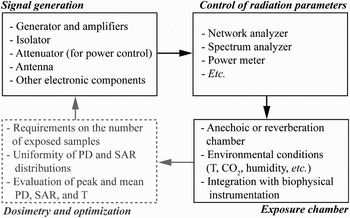
Fig. 8. Schematic representation of a typical millimeter-wave exposure system structure for laboratory studies.
The first sub-unit typically consists of a generator, amplifiers, isolator, attenuator, and radiating structure/antenna (e.g. [Reference Zhadobov26]). The state of the art power limits are delivered by military systems (up to several MW at 94 GHz) [Reference Debouzy, Crouzier, Dabouis, Malabiau, Bachelet and Perrin27]; however, the output power of the generators used for the research purposes at 60 GHz is typically limited to several watts [Reference Zhadobov26, Reference Kurogi, Suzuki and Taki28, Reference Alekseev, Gordiienko, Radzievsky and Ziskin29].
The second sub-unit ensures real-time monitoring of the signal spectrum and output power stability that might be critical for the reliable interpretation of the biological outcome. It consists of a network analyzer, spectrum analyzer, and/or power meter.
Finally, the exposure chamber ensures appropriate environmental conditions for the biological samples under test and determines the EM boundary conditions. The requirements on the environmental conditions depend on the sample type. Biophysical samples, as for instance artificial models of biological membranes [Reference Zhadobov, Sauleau, Vié, Himdi, Le Coq and Thouroude30], require high purity of the air and absence of vibrations. For cells in culture, precise temperature control (37 ± 1°C) and CO2 concentration of 5% must be ensured. The most commonly used animal models (e.g. mice, rats, rabbits [Reference Bellossi, Dubost, Moulinoux, Ruelloux, Himdi and Rocher31]) require air circulation, lighting, and minimization of presence of additional environmental stresses.
Free-space exposure set-ups or anechoic chambers have been previously used in most of the studies implying cell or animal exposures to millimeter waves [Reference Chen, Zeng, Lu and Chiang32–Reference Szabo, Manning, Radzievsky, Wetzel, Rojers and Ziskin35]. Recently, several research projects have been focused on the development of compact millimeter-wave reverberation chambers providing uniform exposure of animals independently on their position.
B) Dosimetry
Dosimetry studies have to be conducted to control and characterize the exposure levels within the samples and to optimize the exposure conditions (uniformity of the field distribution, number of simultaneously exposed samples, etc.). Two complementary approaches have been implemented.
1) NUMERICAL DOSIMETRY
A flowchart illustrating the general principle of the numerical E-field-based dosimetry for bioelectromagnetic studies is presented in Fig. 9. The major challenges for numerical dosimetry at millimeter waves are the following: (1) electrically large problems (λ skin varies from 2.5 to 1.25 mm in 30–100 GHz range; this implies small mesh cell sizes of the numerical models – in the order of 0.1 mm [Reference Zhadobov26]), (2) uncertainties on the precise values of the dielectric properties of tissues and absence of well-established database beyond 20 GHz [Reference Gabriel, Lau and Gabriel14], and (3) multi-scale problems related to the presence of the electrically small sub-structures (e.g. cell monolayers or different layers of skin [Reference Zhadobov, Sauleau, Le Dréan, Alekseev and Ziskin19]). Furthermore, the EM problem should be coupled with the thermodynamic one to carefully take into account possible heating and dielectric constant variations related to the thermal gradients [Reference Cueille, Collin, Pivain and Leveque36]. Significant changes of permittivity values of biological tissues and solutions typically appear for the temperature gradients ΔT > 3°C.
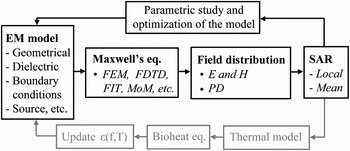
Fig. 9. Schematic representation of the numerical dosimetry approach at millimeter waves.
2) EXPERIMENTAL DOSIMETRY
At millimeter waves, the direct field-based dosimetry faces two major problems. First, the gradients of PD and SAR values within biological tissues are high because of the strongly localized absorption. This implies that the measurements should have spatial resolution better than 0.1–0.2 mm. Second, the already-existing E-field probes are too big in size for the local dosimetry [Reference Pakhomov, Prol, Mathur, Akyel and Campbell37], and additionally may perturb the EM field and temperature distribution. Furthermore, the sample conductivity should be known to determine the local or average SAR. Therefore, this experimental technique has a limited practical interest.
An alternative solution consists in remotely or invasively measuring the near-surface thermal dynamics of the sample under test (2). This is the most efficient way to experimentally determine the SAR and T distributions. Some non-perturbing techniques have been reported for the simultaneous determination of T and SAR, including optical fiber measurements [Reference Zhadobov, Sauleau, Thouroude, Nicolas Nicolaz, Le Quément and Le Dréan38], high-resolution infrared imaging [Reference Zhadobov, Sauleau, Le Dréan, Alekseev and Ziskin19], and utilization of thermosensitive liquid crystals [Reference Suzuki39].
C) Far-field exposures
In most of the reported in vitro studies, a directive antenna is used for the exposure of biological samples under free-space conditions or within a thermo-controlled incubator [Reference Zhadobov26, Reference Zhadobov, Sauleau, Vié, Himdi, Le Coq and Thouroude30, Reference Safronova, Gabdoulkhakova and Santalov33]. In this configuration, several samples can be exposed simultaneously with a sufficient uniformity of the PD distribution (typically better than 30%) and low peak-to-mean PD ratio. The detailed analysis of the PD and SAR distribution within the sample under test is performed using numerical techniques assuming the incident wave to be a normally incident plane wave [Reference Zhao and Wei40]. Finite-difference time-domain (FDTD), finite element method (FEM), and finite integration technique (FIT) have been successfully applied for the numerical dosimetry studies [Reference Zhadobov, Sauleau, Le Dréan, Alekseev and Ziskin19, Reference Zhao and Wei40]. This numerical modeling strategy was validated experimentally using infrared thermometry [Reference Zhadobov, Sauleau, Le Dréan, Alekseev and Ziskin19]. It is worthwhile to note that the meniscus effect, considered as an important factor for frequencies around 1 GHz [Reference Schuderer and Kuster41], can be neglected in bioelectromagnetic studies at millimeter wave.
Several far-field exposure scenarios have also been reported for in vivo studies [Reference Kues, D'Anna, Osiander, Green and Monahan11, Reference Bellossi, Dubost, Moulinoux, Ruelloux, Himdi and Rocher31, Reference Gapeyev, Mikhailik and Chemeris42–Reference Sypniewska, Millenbaugh, Kiel, Blystone, Ringham, Mason and Witzmann44]. Here again, the choice of the far-field exposure conditions was motivated by the more uniform PD distribution compared to near-field exposures. Significant efforts have been undertaken by Alexeev et al. to supply accurate analytical, numerical, and experimental dosimetry for both animal and human skin exposures [Reference Alekseev, Radzievsky, Logani and Ziskin20, Reference Alekseev and Ziskin24, Reference Alekseev and Ziskin25, Reference Alekseev, Gordiienko and Ziskin45]. Infrared radiometry in combination with micro-encapsulated thermosensitive liquid crystals was efficiently used for the EM and thermal dosimetry of rabbit and primates eyes [Reference Kues, D'Anna, Osiander, Green and Monahan11, Reference Suzuki39].
D) Near-field exposures
The major disadvantage of the far-field exposure systems is their low exposure efficiency (typically 10–15%) resulting in low PD levels on the biological samples. For a given antenna, there are two possible solutions to reach higher PD: (1) to increase the output power and (2) to bring closer the antenna and the sample. The first solution requires the use of high-power generators [Reference Jauchem, Ryan and Frei43, Reference Sypniewska, Millenbaugh, Kiel, Blystone, Ringham, Mason and Witzmann44] that are not always available for research purposes [Reference Chen, Zeng, Lu and Chiang46, Reference Nicolas Nicolaz47]. The second solution implies characterization of the near-field distribution in the presence of biological samples in the reactive zone [Reference Zhadobov, Sauleau, Thouroude, Nicolas Nicolaz, Le Quément and Le Dréan38]. It was demonstrated that accurate dosimetry is crucial, as significant variations in T and SAR profiles appear for the displacements of the order of 0.5 mm under near-field exposure conditions [Reference Suzuki, Shibuya, Kurogi, Taguchi, Suzuki and Taki48].
A few near-field configurations have been implemented mainly in studies dealing with the potential therapeutic applications of millimeter waves including exposures at 61 GHz [Reference Alekseev and Ziskin24]. An experimental approach based on a high-resolution infrared imaging has shown to be highly effective for in vivo dosimetry applications [Reference Kurogi, Suzuki and Taki28, Reference Suzuki, Shibuya, Kurogi, Taguchi, Suzuki and Taki48], but also for antenna measurements including determination of the near-field PD distribution [Reference Khizhnyak and Ziskin49]. Near-field exposures were also developed in a number of in vitro studies providing PD up to 100 mW/cm2 at 60 GHz [Reference Szabo, Manning, Radzievsky, Wetzel, Rojers and Ziskin35, Reference Pakhomov, Prol, Mathur, Akyel and Campbell37, Reference Chen, Zeng, Lu and Chiang46, Reference Yu50].
IV. BIOLOGICAL EFFECTS OF MILLIMETER WAVES
There is a growing concern about possible health and biological effects of wireless technologies. Most of the bioelectromagnetic studies have investigated mobile phone exposures, and only few studies have focused on the biocompatibility of millimeter waves. Studies performed before 1998 were summarized by Pakhomov et al. [Reference Pakhomov, Akyel, Pakhomova, Stuck and Murphy51]. In this paper we focus on major studies, essentially at 60 GHz, published after 1998.
Millimeter waves are non-ionizing radiations with a quantum of energy of 1.2 × 10−4–1.2 × 10−3 eV. This energy is not sufficient to break chemical bonds as the energy required to induce ionization is of several orders of magnitude higher (typically 10 eV). However, some processes require much less energy to be altered, for instance the energy required for excitation of rotational modes is 10−5–10−2 eV.
At 10–100 GHz, heating is the major effect resulting from the absorption of the EM energy. Significant thermal responses (ΔT > 0.5°C) appear after exposure to PD > 5 mW/cm2, and, in case of very high-power exposures (above several hundreds of mW/cm2), it can lead to a pain sensation or tissue damage. The expected PD values for the emerging wireless communication systems are low enough to do not induce any biologically significant thermal effect typically appearing for the temperature increments exceeding 1–2°C [Reference Smulders9]. The absence of direct or combined biological effects that do not directly depend on temperature rise is still controversial.
An argument in favor of possible interactions of millimeter waves with living organisms is their therapeutic applications introduced in some Eastern European countries more than 20 ears ago [Reference Rojavin and Ziskin52]. It is still unclear if the observed effects can be fully explained in the framework of the thermodynamics, or if direct EM interference with biological processes might also take place. It is important to underline that, in general, this therapeutic technique is not accepted by the Western medical community.
A) Biological effects at physiological levels
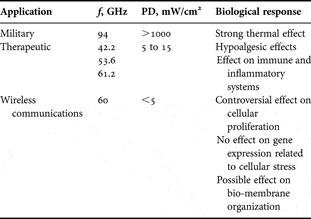
Most of the previous studies related to the interaction of millimeter waves with biological tissues were motivated by their potential therapeutic applications. The operating frequencies and power densities used for these applications are within 42–61 GHz and 5–15 mW/cm2 ranges, respectively. According to the scientific literature, millimeter-wave action could have two main pathways: (1) analgesic effect and (2) effect on the inflammatory and immune systems (Table 3).
Table 3. Summary of the main reported biological effects.
1) STUDIES OF ANALGESIC/HYPOALGESIC EFFECTS
Application of millimeter waves for the pain therapy showed some positive results [Reference Usichenko, Edinger, Gizhko, Lehmann, Wendt and Feyerherd53]. Studies on animals demonstrated that the optimal effect was obtained at 61.22 GHz and 13.3 mW/cm2 [Reference Radzievsky, Gordiienko, Alekseev, Szabo, Cowan and Ziskin54]. Various scientific publications reported positive data using blind tests with animal or volunteer studies. A 15-min exposure allows mice to better withstand noxious stimulation caused by the cold water tail-flick test [Reference Radzievsky, Rojavin, Cowan, Alekseev, Radzievsky and Ziskin55, Reference Rojavin, Radzievsky, Cowan and Ziskin56]. The hypoalgesic effect is not observed for PD < 0.5 mW/cm2 [Reference Rojavin, Radzievsky, Cowan and Ziskin56]. Pretreatment with specific opioid antagonists completely blocked this effect [Reference Rojavin and Ziskin57] that suggests involvement of endogenous opioids (natural molecules involved in pain tolerance).
Taking into account local energy absorption, the most intriguing question is the link of observed effects with the exposure site. Hypoalgesic effects were found in animals and humans exposed at the acupuncture points [Reference Lysenyuk, Samosyuk, Kulikovich and Kozhanova58–Reference Usichenko, Ivashkivsky and Gizhko60]. Moreover, it was found that the most pronounced results were obtained by exposing the skin areas with the high nerve endings concentration [Reference Radzievsky, Rojavin, Cowan, Alekseev and Ziskin61] confirming the role of the peripheral neural system. Stimulation of the neurons located in skin induces a coordinated set of physiologic actions called systemic response (Fig. 10).

Fig. 10. Model illustrating possible mechanisms of millimeter-wave effects.
Two models could explain the therapeutic effects of millimeter waves: (1) direct activation of skin cells (keratinocytes and/or mastocytes) that induces the secretion of molecular signaling factors in the general blood circulation and (2) stimulation of the peripheral neural system that in turn activates the central neural system and induces the secretion of opioids peptides [Reference Radzievsky, Gordiienko, Alekseev, Szabo, Cowan and Ziskin54].
2) EFFECT ON INFLAMMATORY AND IMMUNE SYSTEM
During the past 25 years, the effects of millimeter waves on the immune system have been extensively studied, showing that they can modulate immune responses [Reference Rojavin and Ziskin52].
Szabo et al. analyzed the effect of 61.2-GHz exposure on epidermal keratinocytes by measuring the release of molecules playing a crucial role in cell trafficking in many physiological and pathological processes, so called chemokines (Fig. 10). In this work, the authors have found no modulation of production of the two chemokines involved in inflammatory skin diseases, namely RANTES (regulated on activation and normal T cell expressed and secreted) and IP-10 (interferon-gamma-inducible 10 kDa protein) [Reference Szabo, Manning, Radzievsky, Wetzel, Rojers and Ziskin35, Reference Szabo, Rojavin, Rogers and Ziskin62]. Nevertheless, they showed a modest increase in the intracellular level of IL-1β, a major pro-inflammatory cytokine produced and release by keratinocytes in response to various stimuli [Reference Szabo, Rojavin, Rogers and Ziskin62]. This result suggests that 61.2-GHz exposure could activate keratinocytes. Moreover, the in vivo study of Makar et al. [Reference Makar, Logani, Szabo and Ziskin63, Reference Makar, Logani, Bhanushali, Alekseev and Ziskin64] on mice irradiated at 61.3 GHz and 31 mW/cm2 also showed a pro-inflammatory effect initiated by activation of free nerve endings in the skin.
On the other hand, low-intensity 42-GHz exposure were described as displaying anti-inflammatory actions [Reference Lushnikov, Shumilina, Yakushina, Gapeev, Sadovnikov and Chemeris65, Reference Gapeev, Lushnikov, Shumilina and Chemeris66]. Using a model of local acute inflammation in mice, Gapeyev et al. [Reference Gapeyev, Mikhailik and Chemeris42] have shown that millimeter waves reduces both the footpad edema and local hyperthermia induced by zymosan.
B) Biological effects at cellular and molecular levels
Cellular and sub-cellular experiments have been carried out in order to decipher the molecular mechanisms of possible millimeter-wave interactions. These studies are highly heterogeneous and few of them analyze common biological functions. However, three main topics can be extracted from the scientific literature: (1) effect on cell cycle and proliferation, (2) effect on gene expression, and (3) effect on biological membranes (Table 3).
1) EFFECT ON CELLULAR PROLIFERATION
Millimeter waves have been used in association with conventional drug therapy to treat skin melanoma [Reference Rojavin and Ziskin52] for investigating potential anti-proliferative effects. It was observed that 52–78 GHz exposure reduces the proliferation of human melanoma cells [Reference Beneduci, Chidichimo, De Rose, Filippelli, Straface and Venuta67]. It was also shown that the inhibition of the proliferation was modest and correlated with structural changes and modification of the energy metabolism of the exposed cells [Reference Beneduci, Chidichimo, Tripepi, Perrotta and Cufone68]. However, 2 years latter, the same author failed to reproduce these results [Reference Beneduci69].
On the contrary, studies conducted by the authors' research team suggested that the proliferation was not significantly affected by low-power millimeter-wave exposure [Reference Zhadobov26]. Results coming from Ziskin's research group demonstrated that the anti-cancer properties of millimeter waves may be indirect. It was found that exposure can reduce tumor metastasis through activation of natural killer cells [Reference Logani, Szabo, Makar, Bhanushali, Alekseev and Ziskin70], or protect cells from the toxicity of commonly used anticancer drugs [Reference Makar, Logani, Szabo and Ziskin63].
2) EFFECT ON GENETIC EXPRESSION
According to the non-ionizing nature of the millimeter waves, most of the studies reported that they are not genotoxic [Reference Vijayalaxmi, Logani, Bhanushali, Ziskin and Prihoda71]. On the other hand, the hypothesis of possible proteotoxic effect has been issued. Recently, our group published a thorough study showing that, if care is taken to avoid thermal effects, no notable change of protein chaperone expression, such as HSP70 or clusterin, could be detected [Reference Zhadobov72]. These results are in agreement with previously reported data [Reference Szabo, Manning, Radzievsky, Wetzel, Rojers and Ziskin35], which demonstrated the absence of the effect on the HSP induction. In order to further explore the potential proteotoxic effect of millimeter waves, we analyzed the effect on the reticulum stress and its associated unfolded protein response. Our results demonstrated that 59–61.2 GHz radiations at 0.14 mW/cm2 do not affect the endoplasmic reticulum homeostasis [Reference Nicolas Nicolaz47, Reference Nicolas Nicolaz73]. These data indicate that millimeter waves do not trigger an acute stress necessitating a transcriptional response.
3) EFFECT ON BIOMEMBRANES
Cell membrane consists of phospholipid bilayer with embedded proteins. Its structure is dynamic, although highly ordered, and several studies suggested that it may be a molecular target of millimeter waves. For instance, data from our group showed that 60 GHz exposure at PD levels close to those typically expected from wireless communication systems (0.9 mW/cm2) can induce structural modifications in artificial biomembranes [Reference Zhadobov, Sauleau, Vié, Himdi, Le Coq and Thouroude30]. The exposure reversibly increased the lateral pressure of the phospholipid monolayer, but this event is not strong enough to disturb the phospholipid microdomain organization within a biomembrane.
In parallel, it was demonstrated that structural changes in cellular membranes may occur during the exposure at 42.2 GHz (35.5 mW/cm2) [Reference Szabo, Kappelmayer, Alekseev and Ziskin74]. Reversible externalization of phosphatidylserine was observed without cellular damage that can eliminate the hypothesis of apoptosis induction. The biological relevance of this observation is not clear, but such a modification could play a role in the cellular signaling or cellular interactions. Moreover, it was demonstrated that 53-GHz radiations and 130-GHz pulse-modulated exposures can induce physical changes and modify permeability of phospholipid vesicles [Reference Ramundo-Orlando75, Reference Ramundo-Orlando76]. It was assumed that millimeter waves could interfere with the orientation of charged and dipolar molecules leading to changes at the membrane/water interface.
V. CONCLUSION
This paper summarizes the most significant recent results and advances in the field of interaction of millimeter waves with the human body. EM, thermal, and biological aspects are reviewed with a particular emphasis on 60-GHz exposures. The recently introduced dosimetric techniques and instrumentation for bioelectromagnetic laboratory studies are presented.
First, available data on the dielectric properties of skin at 60 GHz is summarized demonstrating that well-established permittivity database is missing for the millimeter-wave band. It is shown that 26–41% of power is reflected at the air/skin interface for the normal incidence, and this value deviates significantly for illuminations under oblique incidence. More than 90% of the transmitted power is absorbed by the skin, and therefore single- or multi-layer skin model is sufficient for the reliable EM dosimetry. Clothing in direct contact with the skin enhances the power transmission, whereas an air gap of 0–2 mm between the clothes and skin decreases the transmission. Millimeter waves induce steady-state temperature increments of the order of several tenths of °C for PD below the current far-field exposure limits; however, significant thermal effects may appear for local near-field exposures. It was demonstrated that, in contrast to the EM model, for the reliable thermal dosimetry, it is crucial to consider multi-layer structure including skin, fat, and muscle.
In addition, the state of the art in the development of laboratory exposure set-ups at millimeter waves, as well as in the numerical and experimental millimeter-wave dosimetry is provided in order to highlight the significant progress in this field during the last decade. An exhaustive analysis of the in vitro and in vivo biological studies is performed for the low- and high-power exposures. Whereas some effects have been observed for the medium-power exposures (5–15 mW/cm2) leading to the local heating of the order of 1–2°C, most of the reproducible results for the lower PD demonstrated that direct biological effects at 60-GHz are not likely. Future trends of the bioelectromagnetic studies at millimeter waves cover such aspects as investigation of possible synergistic and combined EM/thermal effects, exact determination of power thresholds, and identification of possible biomarkers of the millimeter-wave exposure.
Finally, new trends in wireless BAN suggest that millimeter-wave on- and off-body sensors including wearable antennas are of increasing interest. To design appropriate antennas for these applications, the influence of the body on the antenna performances should be carefully taken into account. Therefore an accurate characterization of skin dielectric properties is required. Besides, one of the promising currently unexplored solutions to reduce susceptibility to shadowing is to implement reconfigurable millimeter-wave wearable antennas. For on-body applications, the characterization of the propagation channel is of utmost importance as it significantly differs from the free-space one, and no information is currently available in the scientific literature on this issue.
ACKNOWLEDGEMENTS
This work was supported by Agence Nationale de la Recherche (ANR), France, under ANR-09-RPDOC-003–01 grant (Bio-CEM project) and by “Centre National de la Recherche Scientifique (CNRS)”, France.
 Maxim Zhadobov received the M.S. degree in radiophysics from Nizhni Novgorod State University, Russia, in 2003, and the Ph.D. degree in bioelectromagnetics from Institute of Electronics and Telecommunications of Rennes (IETR), France, in 2006. He accomplished post-doctoral training at the Center for Biomedical Physics, Temple University, Philadelphia, USA, in 2008 and then rejoined IETR as an Associate Scientist CNRS. His main scientific interests are in the field of biocompatibility of electromagnetic radiations, including interactions of microwaves, millimeter waves, and pulsed radiations at the cellular level, health risks, and environmental safety of emerging wireless communication systems, biocompatibility of wireless biomedical techniques, therapeutic applications of non-ionizing radiations, bioelectromagnetic optimization of body-centric wireless systems, experimental and numerical electromagnetic dosimetry. Dr. Zhadobov was the recipient of the 2005 Best Poster Presentation Award from the International School of Bioelectromagnetics, 2006 Best Scientific Paper Award from the Bioelectromegnetics Society, and Brittany's Young Scientist Award in 2010.
Maxim Zhadobov received the M.S. degree in radiophysics from Nizhni Novgorod State University, Russia, in 2003, and the Ph.D. degree in bioelectromagnetics from Institute of Electronics and Telecommunications of Rennes (IETR), France, in 2006. He accomplished post-doctoral training at the Center for Biomedical Physics, Temple University, Philadelphia, USA, in 2008 and then rejoined IETR as an Associate Scientist CNRS. His main scientific interests are in the field of biocompatibility of electromagnetic radiations, including interactions of microwaves, millimeter waves, and pulsed radiations at the cellular level, health risks, and environmental safety of emerging wireless communication systems, biocompatibility of wireless biomedical techniques, therapeutic applications of non-ionizing radiations, bioelectromagnetic optimization of body-centric wireless systems, experimental and numerical electromagnetic dosimetry. Dr. Zhadobov was the recipient of the 2005 Best Poster Presentation Award from the International School of Bioelectromagnetics, 2006 Best Scientific Paper Award from the Bioelectromegnetics Society, and Brittany's Young Scientist Award in 2010.
 Nacer Chahat graduated in electrical engineering and radio communications from the Ecole Supérieur d'ingénieurs de Rennes (ESIR), received the master's degree in telecommunication and electronics in 2009. Since 2009, he has been working toward the Ph.D. degree in signal processing and telecommunications at the Institute of Electronics and Telecommunications of Rennes (IETR), University of Rennes 1, Rennes, France. His current research fields are millimeter-wave antennas, and the evaluation of the interaction between the electromagnetic field and human body. He accomplished a 6-month master's training period as a special research student in 2009 at the Graduate School of Engineering, Chiba University, Chiba, Japan.
Nacer Chahat graduated in electrical engineering and radio communications from the Ecole Supérieur d'ingénieurs de Rennes (ESIR), received the master's degree in telecommunication and electronics in 2009. Since 2009, he has been working toward the Ph.D. degree in signal processing and telecommunications at the Institute of Electronics and Telecommunications of Rennes (IETR), University of Rennes 1, Rennes, France. His current research fields are millimeter-wave antennas, and the evaluation of the interaction between the electromagnetic field and human body. He accomplished a 6-month master's training period as a special research student in 2009 at the Graduate School of Engineering, Chiba University, Chiba, Japan.
 Ronan Sauleau graduated in 1995 from the “INSA de Rennes”, Rennes, France. He joined the “Ecole Normale Supérieure de Cachan” in 1995 and received the “Agrégation” in 1996. He received the Ph.D. degree from the University of Rennes 1 in 1999. He received the “Habilitation à Diriger des Recherches” in November 2005 and is Full Professor since November 2009. His main fields of interest are millimeter-wave antennas, focusing devices, periodic structures (EBG materials and metamaterials), and biological effects of millimeter waves. He holds five patents and has co-authored 83 journal papers and more than 200 publications in national and international conferences. He received the 2004 ISAP Young Researcher Scientist Fellowship (Japan) and the first Young Researcher Prize in Brittany, France in 2001. In 2007, he was elevated as a Junior Member of the “Institut Universitaire de France”. He received the Bronze Medal from CNRS in 2008.
Ronan Sauleau graduated in 1995 from the “INSA de Rennes”, Rennes, France. He joined the “Ecole Normale Supérieure de Cachan” in 1995 and received the “Agrégation” in 1996. He received the Ph.D. degree from the University of Rennes 1 in 1999. He received the “Habilitation à Diriger des Recherches” in November 2005 and is Full Professor since November 2009. His main fields of interest are millimeter-wave antennas, focusing devices, periodic structures (EBG materials and metamaterials), and biological effects of millimeter waves. He holds five patents and has co-authored 83 journal papers and more than 200 publications in national and international conferences. He received the 2004 ISAP Young Researcher Scientist Fellowship (Japan) and the first Young Researcher Prize in Brittany, France in 2001. In 2007, he was elevated as a Junior Member of the “Institut Universitaire de France”. He received the Bronze Medal from CNRS in 2008.
 Catherine Le Quément received the Ph.D. degree in biology from the University of Rennes 1, Rennes, France in 2008. From 2006 to 2008, she was an Associate Professor at the University of Rennes 1 (Attachée Temporaire d'Enseigment et de Recherche), where she taught pharmacology and biochemistry. Since 2008, she accomplishes post-doctoral training at the Cellular and Molecular Interactions lab, Rennes, France. Her main subject of interest is cell responses to environmental stress (cigarette smoke, eletromagnetic waves, etc.).
Catherine Le Quément received the Ph.D. degree in biology from the University of Rennes 1, Rennes, France in 2008. From 2006 to 2008, she was an Associate Professor at the University of Rennes 1 (Attachée Temporaire d'Enseigment et de Recherche), where she taught pharmacology and biochemistry. Since 2008, she accomplishes post-doctoral training at the Cellular and Molecular Interactions lab, Rennes, France. Her main subject of interest is cell responses to environmental stress (cigarette smoke, eletromagnetic waves, etc.).
 Yves Le Dréan received the Ph.D. degree and “Habilitation à Diriger des Recherches” in biology from the University of Rennes 1, Rennes, France in 1993 and 2007, respectively. He joined the hospital for sick children at Toronto, Canada, as a post-doctoral fellow in 1994. Since 1997, he has been an Associate Professor at the University of Rennes 1, where he teaches molecular biology and biochemistry. His main subject of interest is the control of genetic expression. His current research activities are related to the investigations of cell responses to proteotoxic stress. Since 2004 he has been also actively involved in the field of biological effects of electromagnetic waves.
Yves Le Dréan received the Ph.D. degree and “Habilitation à Diriger des Recherches” in biology from the University of Rennes 1, Rennes, France in 1993 and 2007, respectively. He joined the hospital for sick children at Toronto, Canada, as a post-doctoral fellow in 1994. Since 1997, he has been an Associate Professor at the University of Rennes 1, where he teaches molecular biology and biochemistry. His main subject of interest is the control of genetic expression. His current research activities are related to the investigations of cell responses to proteotoxic stress. Since 2004 he has been also actively involved in the field of biological effects of electromagnetic waves.