Many HTA bodies explicitly support decision-making processes, such as technology reimbursement/coverage decisions, for which timely information is required (Reference Cranovsky, Matillion and Banta9). As a result of this, many HTAs adopt a pragmatic approach that focuses on a subset of the elements of HTA, such as clinical effectiveness and cost-effectiveness. Whereas the broader aspects of HTA, such as ethical, social, legal, organizational, and other system impacts of the diffusion of the technology, may still be considered in the assessment, often these are not addressed within a transparent and explicit framework and are less likely to be evidence-based to the same extent (Reference Watt, Cameron and Sturm34).
Differences in the content and application of HTA have raised the question of whether some degree of harmonization of HTA between countries would be worthwhile (4–Reference Banta6;Reference Jonsson, Banta, Henshall and Sampietro-Colom24;Reference Sassi32). The concept of improving efficiency across assessment bodies has been central to this; that is harmonization might avoid duplication of effort on the part of both manufacturers and decision makers. This has been the subject of debate, much of which has been of a general nature and often based on the premise that harmonization per se is both desirable and feasible. This study adopts an alternative position and questions the underlying premise that harmonization is both desirable and feasible. The analysis focuses on the use of HTA in the context of reimbursement and coverage decisions, as described below.
HTA IN THE CONTEXT OF REIMBURSEMENT AND COVERAGE DECISIONS
Whereas the use of HTA to inform health policy making began in the 1970s, its profile increased among all stakeholders in the 1990s when it was used to inform decisions regarding the reimbursement and coverage of drugs and medical devices (Reference Henry18), which necessitated the adoption of a more transparent and explicit approach. Following application in Australia (29) and Canada (Reference Detsky11), the organizations supporting reimbursement decision making in Western European countries have adopted some form of HTA to help inform reimbursement and coverage decisions.
Figure 1 is intended to represent the stages of a reimbursement decision-making process that incorporates HTA. The institutions and processes through which these stages are carried out vary considerably between countries as they must be considered in the context of the over-arching values and priorities of the society, the form of decision-making structures, and the resources available. As a result, even if all countries used identical assessment methods and a similar evidence base, they would not necessarily reach the same conclusion regarding a particular technology, because of differing values and preferences. Variation in decisions between jurisdictions is to be expected. What is important is that the processes by which the decisions are reached, and the evidence on which they are based, are sufficiently transparent to allow the reasons for differences in HTA recommendations to be understood.
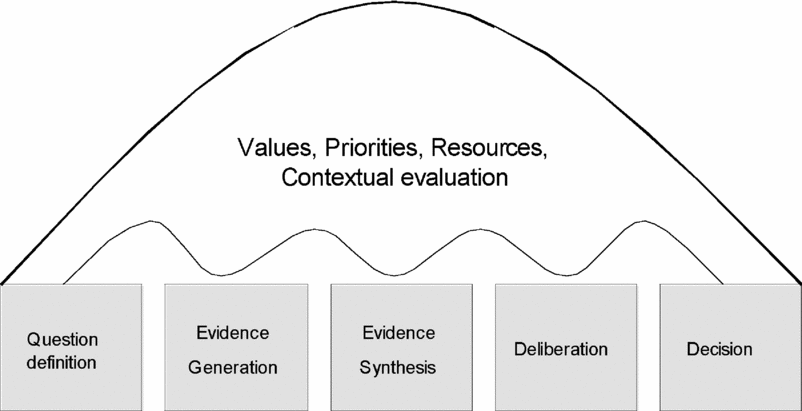
Figure 1. Stages of a reimbursement decision process.
The remainder of this study focuses on the more objective aspects of HTA, namely, the evidence requirements, and considers the degree to which harmonization of these is feasible and desirable. This study makes no attempt to explore the extent to which the more subjective aspects of the HTA process, such as the decision-making process and the development of recommendations, can be harmonized.
THE RATIONALE FOR HARMONIZATION OF HTA EVIDENCE REQUIREMENTS
A range of possible benefits from harmonization of HTA has been identified, which can be grouped into two broad areas: more efficient use of analytical resources; and faster and more appropriate reimbursement decisions. The extent to which harmonization of evidence requirements in HTA can generate benefits is related to the contextual nature of the evidence. HTA is used to inform decisions in the context of the local healthcare system, and different inputs to HTA may be more or less context-specific (Reference Culyer and Lomas10). For example, the evidence from international clinical studies, or systematic literature reviews of clinical studies, might be considered largely context-free, and applicable in multiple jurisdictions. On the other hand, data on healthcare resource use associated with a technology may be very specific to a particular health system.
Possible disadvantages of harmonization of HTA have also been identified (Reference Banta6). These include the risk of decisions in one country being copied in others, without proper analysis of the contextual factors, simply to save resources. Standardization of HTA methods may also lead to less innovative thinking and slow the pace of development in the academic disciplines contributing to HTA.
The relative benefits of harmonization vary with the perspectives of the main stakeholders. Health service decision makers will be concerned with both the cost and quality of decisions. In any country the decision-making system for the reimbursement of health technologies reflects the historical development of the health service and the political processes of that country (Reference Hutton, McGrath and Frybourg21). It would be inappropriate for a country to economize on time and resources by simply adopting technology decisions made elsewhere, with no adaptation to local circumstances. There may, however, be some benefits from pooling analytical expertise and sharing some of the inputs to HTA, while recognizing the need for context-specific analysis (Reference Banta6). Transparency in the reporting of HTA studies is essential if the possibility of appropriate transfer of results or data is to be identified.
Manufacturers also face advantages and disadvantages in the harmonization of evidence requirements. If there was general agreement between countries on the evidence requirements for HTA, manufacturers would be better able to plan data generation activities. It could also lead to higher quality data if resources were concentrated on fewer, high quality studies. This might lead to faster decisions through the use of data with the minimum of local adaptation, and more effective use of analytical resources by manufacturers and HTA agencies. Manufacturers would also benefit from having more stable HTA requirements, allowing them to ensure that these are taken into account in candidate selection and investment decisions and that appropriate outcomes are incorporated into clinical strategies. Where systems are in a process of rapid development, this can cause difficulties in attempting to interpret what information will be required to support market access at product launch. A concern for manufacturers is the risk that a harmonized approach to HTA might be adopted, but individual countries might continue to require country-specific data and analyses, thereby increasing the evidential burden rather than reducing it.
POTENTIAL FOR HARMONIZATION OF EVIDENCE REQUIREMENTS IN HTA
Evidence and information for HTA are required in three main areas: clinical effectiveness; cost-effectiveness; and ethical, social, and legal issues.
Most HTA agencies approach the evaluation of clinical evidence in a similar manner, considering the quality of the study, potential sources of bias and the validity of the results. Clinical effectiveness evidence is generally considered to be the most context-free, but is not without problems. The main contextual considerations relate to whether the populations studied are similar to the local population and whether the estimated benefits can be reproduced in clinical practice. For example, in moving from measurement of efficacy to true effectiveness context-specific factors, such as compliance and healthcare organization become more influential (Reference Haynes17). Nonetheless, the scope for harmonization of clinical evidence requirements in HTA across jurisdictions is extensive, particularly with regard to systematic reviews of effectiveness.
Conversely, the economic aspects of health technology assessment are inevitably more context-specific. There are significant differences in treatment pathways and healthcare resource unit costs which are incorporated into economic models. Therefore, even if much of the effectiveness data comes from a single source, a separate cost-effectiveness analysis is likely to be required in each jurisdiction. However, there is scope for harmonization of the analytical frameworks used in cost-effectiveness modeling. Conversion of clinical outcomes to economically relevant measures (e.g., utilities) requires the introduction of context-specific preferences, although the comparative studies of European countries using EQ-5D show that inter-country differences may be quite small (Reference Greiner, Weijnen and Nieuwenhuizen14). There may also be some agreement on the epidemiological framework that is the foundation of economic models in a particular indication. For example, evidence from the Framingham cohort has been widely used in economic evaluations of cardiovascular disease (Reference Grieve, Hutton and Green15). Despite being based on a very specific North American population, this has often been used in other jurisdictions on the grounds that it is the best available evidence to inform decision making (Reference Cappuccio, Oakeshott, Strazzullo and Kerry8). Therefore, whereas some aspects of economic evaluation remain highly context-specific, there is scope for further exploration of harmonization of others.
Ethical, social, and legal aspects of HTA appear to have received less systematic attention in individual HTAs, making judgment on the scope for harmonization of these elements more difficult. Although these factors are taken into account by many HTA bodies, their use tends to be implicit rather than explicit, possibly due to slower methodological development in these areas compared with the more quantitative aspects of HTA. However, the ethical, social, and legal principles within which a system of reimbursement decision making operates are often determined not at the individual technology level but at the program level, as they apply to all technologies. Thus, their importance may be underestimated by focusing on individual HTAs. See, for example, the NICE document on social value judgments (28). The importance of these factors in the consideration of individual technologies will vary. For example, an HTA of a maternal screening program for fetal abnormalities is likely to be more explicit about ethical issues than one of a pharmaceutical for a common disease. The form and sources of evidence on these issues may differ from those for costs and effectiveness, but checklists of factors to consider have been published (Reference Hofmann20) and approaches to integrating social and ethical values in the whole HTA process have been proposed (Reference Autti-Rämö and Mäkelä3).
LESSONS FROM PREVIOUS HTA HARMONIZATION ACTIVITIES
Assessment of the benefits of further harmonization of evidence requirements in HTA should take account of previous harmonization projects regarding HTA and other related disciplines.
The European Experience
HTA has been widely implemented throughout European Union (EU) Member States. The common objective of HTA in the EU countries could be summarized as providing decision makers with reliable information concerning the implications of healthcare interventions to allow scientifically based health policy making (Reference Jonsson, Banta, Henshall and Sampietro-Colom24).
The perceived benefits of increased coordination across the region led to a series of EU-funded projects from 1993 to 2002 (Reference Banta and Oortwijn5;Reference Banta, Werko and Cranovsky7;Reference Jonsson, Banta, Henshall and Sampietro-Colom23;Reference Jonsson, Banta, Henshall and Sampietro-Colom24;Reference Sassi32). The recommendations of these harmonization initiatives are being implemented through the EUnetHTA project, which involves HTA Agencies from across Europe. The objectives of the EUnetHTA project were (i) to provide a robust multifaceted input to decision making, (ii) to reduce duplication of work, (iii) to gain a better understanding of the links between HTA and policy making in different Member States, and (iv) to support countries with limited HTA experience. The tools developed include a framework for core HTA evidence, an adaptation toolkit for local healthcare systems and a process to facilitate sharing and production of information on new technologies (13). The aspiration is to make this collaborative work among HTA Agencies permanent from 2009 onward, with an emphasis on co-ordination, facilitation and knowledge transfer, rather than standardization of approaches or centralization of decision making.
In parallel with EUnetHTA, the harmonization of clinical effectiveness has also been addressed in the European Commission Pharmaceutical Forum (http://ec.europa.eu/enterprise/phabiocom/comp_pf_en.htm), which includes representation from a broader range of stakeholders, including industry, political and health system decision makers. As a key stakeholder in HTA and the main provider of evidence for this purpose, it is important that technology manufacturers are engaged in harmonization efforts
The North American Experience
In addition to the European efforts to harmonize HTA activities discussed above, other regions have experience of coordinating HTA across multiple organizations and localities, often with great success. For example, the Academy of Managed Care Pharmacy (AMCP) guidelines in the United States provide a format for HTA submissions which is believed to have been adopted by over fifty private and public healthcare purchasing organizations, generating a common standard for formulary submissions in the United States (Reference Taylor and Drummond33).
Public sector activities in the United States, led by the Agency for Healthcare Research and Quality (AHRQ), have concentrated on standardizing approaches to conducting comparative effectiveness reviews for use in informing evidence-based practice (1).
The Canadian Coordinating Office for Health Technology Assessment (CCOHTA), subsequently known as the Canadian Agency for Drugs and Technologies in Health (CADTH), has fostered co-ordination and harmonization of HTA activities across multiple jurisdictions in Canada (Reference Sanders31). The federal structure of Canada dictates that healthcare is almost entirely managed at a Provincial level, resulting in the development of multiple Provincial HTA bodies. CCOHTA's mandate was to facilitate coordination of activities, avoid unnecessary duplication across these bodies and improve the quality of HTA (Reference Menon and Topfer27). In this respect it has similarities to Europe, and it has been suggested that European harmonization efforts could learn from the Canadian experience (Reference Hailey16;Reference McDaid26).
Experience in Related Processes
Although progress with the harmonization of HTA appears to have been slow, this must be placed in the context of other harmonization initiatives, such as the International Conference on Harmonisation (ICH) in drug regulation (22;Reference Juillet25;Reference Rockfold30). This collaboration, between the United States, the EU, and Japan, produced agreement on data requirements to support many aspects of the application process for drug licensing across the different jurisdictions. During the 1990s, working groups with two representatives from each region (government and an umbrella pharmaceutical industry association) developed guidance to cover all aspects of drug development. A related activity has now begun in medical device regulation; the Global Harmonisation Task Force presents the key concepts related to clinical evidence for a device and guidance on methods of clinical evaluation [http://www.ghtf.org/sg5/]. Key lessons to emerge from the ICH experience are that the process must begin with a clearly defined common goal and a willingness to participate in open dialogue; a period of collaboration and agreement should precede moves to full harmonization and discussions must begin at a detailed technical level, for example to agree on standard terminology, before proceeding to higher level political approval. Harmonization should also be regarded as a continuous process reflecting changes in practice and scientific knowledge, rather than as a discrete activity.
More recently an attempt was made to harmonize the registration processes for health products of Australia and New Zealand (2). Progress was made through technical working groups, and governmental agreements but final implementation did not take place because of lack of political support. This reflected the fact that the systems in the two countries were less similar than had at first been supposed, and that the differences became more apparent as the process progressed. An important factor was that the concerns of the Maori community in New Zealand over the way that traditional medicines would be treated were not recognized at the outset. This, together with other political changes, led to loss of support for the process. There is a clear lesson here that failure to engage a major stakeholder at an early stage can lead to problems later on, if that stakeholder believes that important issues are not being addressed.
WHERE NEXT FOR HARMONIZATION?
If each of the stakeholder groups involved in HTA faces positive and negative effects from the harmonization of evidence requirements and past experience has resulted in limited progress, where should future efforts on harmonization focus? There is now some degree of agreement between researchers and practitioners on the principles of HTA, but not on the details of the methodology and evidence standards (Reference Drummond, Dubois and Garattini12;13;Reference Hjelmgren, Berggren and Andersson19). Opportunities for harmonization of the three main evidence requirements along with HTA processes are presented below.
Clinical Evidence
This area offers the most immediate opportunities for progress because (subject to demographic, epidemiological and other factors) clinical data are generally regarded as transferable across geographical and social boundaries. Activities in the following areas could be of benefit to decision makers and manufacturers of technologies: (i) More agreement on the design of clinical trials in particular disease areas (e.g., in the selection of comparators, use of surrogate endpoints, duration of follow-up, and use of quality of life measures in clinical trials); (ii) More standardization of methods in systematic reviews of clinical evidence (e.g., appropriate inclusion criteria).
Adopting the ICH approach, with agreement on general principles, followed by the development of a specific working group on each topic, could help to further this agenda. Consideration of these aspects should also seek to take account of the scope for harmonization between HTA and regulatory assessment on clinical evidence requirements.
Economic Evidence
The context-specific nature of economic evidence means scope for harmonization is more limited than for clinical evidence. However, on the outcomes side, the harmonization of approaches to the measurement and valuation of health benefits could have advantages. More general agreement on the appropriateness of quality-adjusted life-years (QALYs) as a standardized outcome measure could be explored, before addressing the practical issues of utility measurement itself. Although the valuation of health benefits will be influenced by local cultural and social factors, some aspects of the measurement process are transferable.
Although the issues regarding standardization of economic methods between countries have been intensively researched (Reference Hjelmgren, Berggren and Andersson19), there is scope for greater co-ordination of the analytical framework for appraisals within each particular disease area. For example, the use of a standardized approach to epidemiological modeling of disease processes and treatment pathways could provide a framework for identifying the differences in results when local data are entered into decision analyses. Indications with intensively researched epidemiological models, such as diabetes and cardiovascular disease, would appear to offer an opportunity to test this approach.
Ethical Social and Legal issues
Despite their importance, there is still a need to sensitize decision makers to these issues. Guidelines and checklists on ethical issues in HTA, such as those produced by INAHTA, exist but need to be given a higher profile. However, it should be acknowledged that many of the issues are highly context-specific and informed by sociocultural and legal factors within a particular jurisdiction.
Given the lack of information about these aspects of HTA, there could be value in the explicit mapping of approaches used in different countries and health systems. This might reveal more similarities between jurisdictions than expected. Identical ethical positions would not be expected but a common set of issues to address might be agreed.
Decision Processes
It should be acknowledged that harmonization of evidence requirements cannot be considered independently of the decision process which the evidence is intended to inform. There is an important link between question definition, decision-criteria, and hence evidence requirements. While it is unrealistic to expect identical decision processes and institutions, there might be agreement on the conceptual stages of a decision using HTA, such as those described in Figure 1.
There is also the possibility of agreement on key principles of decision making, for example, transparency, the right of stakeholders to submit evidence, the expectation of an explanation of the basis of a decision, and the right of appeal to an independent authority. However, acceptance of such a set of principles would require the decision makers currently using HTA, as well as the manufacturers and sponsors of technologies, to recognize its value in reaching sound decisions. The disparity of procedures currently in operation indicates that this might not be achievable in the shortterm.
CONCLUSION
There is considerable uncertainty among stakeholders regarding the benefits of harmonization of HTA. In reviewing the possible approaches to harmonization, it was recognized that harmonization of HTA across jurisdictions should not aim to produce a single decision on reimbursement and utilization of a technology. The inherent differences between economies, societies, and health systems mean that such an outcome would be neither feasible nor desirable, even if identical decision processes were used. A more desirable and achievable target might be to be able to justify differences in decisions between jurisdictions by reference to evidence, values, and priorities. Transparency in reporting the bases of decisions will be essential if this is to be achieved.
Overall, the pursuit of harmonization of evidence requirements in HTA across jurisdictions may yield benefit. The possibility of benefit from harmonization of HTA evidence requirements with those of related decision-making processes—particularly product licensing—is worthy of further exploration. Decision makers and industry representatives agree that in trying to reduce duplication of effort in the generation and analysis of data, it is important to retain local ownership of the results of HTA, if it is to have an impact on decision making. It is also important that any attempts to standardize HTA practices do not restrict new developments in methods and processes that diversity of approach can produce.
Of the three types of evidence considered, the most scope for benefit from harmonization is thought to lie in the generation and evaluation of clinical evidence, which is generally believed to be less context-specific. Within the economic domain, the potential benefits of more standardization of analytical frameworks (e.g., agreement on common epidemiological bases for decision-models) is identified as an area for further research. The ethical, social, and legal areas are generally believed to be under-researched. More exploration of the role of these issues, the degree to which they are used explicitly and the methodological frameworks adopted within HTA systems is needed before the value of harmonization can be considered.
In conclusion, further harmonization of evidence requirements across HTA bodies could be beneficial in specific circumstances. There are benefits associated with more exchange of ideas, methods, and data between jurisdictions. However, further harmonization of the use of HTA in reimbursement decisions is not thought to be either desirable or feasible at this time as such decisions should be framed in local social, cultural, and political contexts. Experience of other harmonization initiatives shows that lengthy periods of collaboration and exchange of ideas are necessary to bring the participants to the point of accepting common approaches. It needs to be acknowledged that the role of HTA in decision-making processes remains relatively immature in comparison with other processes, such as regulatory approval. The creation of inclusive networks, for example through EUnetHTA and the European Commission Pharmaceutical Forum, may help to create an environment more favorable to harmonization in the future.
HTAi POLICY FORUM PARTICIPANTS
The participants are as follows: Dave Ames, Johnson and Johnson, Canada; Bert Boer, CVZ, The Netherlands; Matthew Brougham, PHARMAC, New Zealand (invited by CADTH, Canada); Nick Bruce, Pfizer, United Kingdom; Kathy Cargill, Medtronic, Switzerland; Frances Charlesworth, AstraZeneca, United Kingdom; Peter de Jong, Cordis Europe, Johnson and Johnson, Belgium; Catherine Farrell, MSAC, Australia; Wim Goettsch, CVZ, The Netherlands; Alicia Granados, Scientific Advisory Board of CIRIT, Government of Catalonia, Spain; Jens Grueger, Novartis, Switzerland; Neil Johnson, AstraZeneca, United Kingdom; Hans Jörg Fugel, MerckSerono, Switzerland; Brendon Kearney, MSAC, Australia; Jeff Kirsch, GlaxoSmithKline, United Kingdom; Marguerite Koster, Kaiser Permanente, USA; Carole Longson, NICE, United Kingdom; Caroline Lowe, Merck, USA; Clare McGrath, Pfizer, United Kingdom; Andrew Mitchell, PBAC, Australia; Sandy Pagotto, CADTH, Canada; Andrea Rappagliosi, MerckSerono, Switzerland; Sir Michael Rawlins, NICE, United Kingdom; Milena Richter, GlaxoSmithKline, United Kingdom; Lloyd Sansom, PBAC, Australia; Jean Slutsky, AHRQ, USA; Franz Waibel, Merck, USA.
Invited Participants are as follows: Hilda Bastian, Invited Participant, IQWiG, Germany; Stephen Dellar, Invited Participant, Pharmaceutical Evaluation Branch, Australia; Chris Henshall, Invited Participant, University of York, United Kingdom; Steve Pearson, Invited Participant, ICER, Harvard Medical School, USA; John Arne Røttingen, Invited Participant, NOKC, Norway
Board Members are as follows: Reiner Banken, HTAi Board President, Canada; Jean Francois Baladi, HTAi Board Director, USA; Americo Cicchetti, HTAi Board Director, Italy; Clifford Goodman, HTAi Board Director, USA; Guy Maddern, HTAi Board Director, Australia; Berit Morland, HTAi Board Director, Norway; Wija Oortwijn, HTAi Board Director, The Netherlands; R M Padney, HTAi Board Observer for Developing Countries, India; Alric Ruther, HTAi Board Observer for INAHTA, Germany; Laura Sampietro-Colom, HTAi Board Director, Spain; John Sproule, HTAi Board Observer for Secretariat, Canada; Ken Stein, HTAi Board Director, United Kingdom; Mitchell Sugarman, HTAi Board Director, USA.
CONTACT INFORMATION
John Hutton, Bphil (jh602@york.ac.uk), Professor, Paul Trueman, MSc (pt507@york.ac.uk), Director, York Health Economics Consortium, University of York, Market Square, Heslington, York YO10 5NH, UK
Karen Facey, PhD (k.facey@btinternet.com), Chair, HTAi Policy Forum, Woodlands Lodge, Buchannan Castle Estate, Drymen G63 0HX, UK



