Incorporating environmental and sustainability considerations into health technology assessment and clinical and public health guidelines: a scoping review
Published online by Cambridge University Press: 13 December 2022
Abstract
Healthcare systems account for a substantial proportion of global carbon emissions and contribute to wider environmental degradation. This scoping review aimed to summarize the evidence currently available on incorporation of environmental and sustainability considerations into health technology assessments (HTAs) and guidelines to support the National In stitute for Health and Care Excellence and analogous bodies in other jurisdictions developing theirown methods and processes. Overall, 7,653 articles were identified, of which 24 were included in this review and split into three key areas – HTA (10 studies), healthcare guidelines (4 studies), and food and dietary guidelines (10 studies). Methodological reviews discussed the pros and cons of different approaches to integrate environmental considerations into HTAs, including adjustments to conventional cost-utility analysis (CUA), cost–benefit analysis, and multicriteria decision analysis. The case studies illustrated the challenges of putting this into practice, such as lack of disaggregated data to evaluate the impact of single technologies and difficulty in conducting thorough life cycle assessments that consider the full environmental effects. Evidence was scant on the incorporation of environmental impacts in clinical practice and public health guidelines. Food and dietary guidelines used adapted CUA based on life cycle assessments, simulation modeling, and qualitative judgments made by expert panels. There is uncertainty on how HTA and guideline committees will handle trade-offs between health and environment, especially when balancing environmental harms that fall largely on society with health benefits for individuals. Further research is warranted to enable integration of environmental considerations into HTA and clinical and public health guidelines.
Keywords
- Type
- Article Commentary
- Information
- Creative Commons
- This is an Open Access article, distributed under the terms of the Creative Commons Attribution licence (http://creativecommons.org/licenses/by/4.0), which permits unrestricted re-use, distribution and reproduction, provided the original article is properly cited.
- Copyright
- © The Author(s), 2022. Published by Cambridge University Press
Footnotes
A.-C.P.-G. and S.-H.Y. are joint first authors.
References
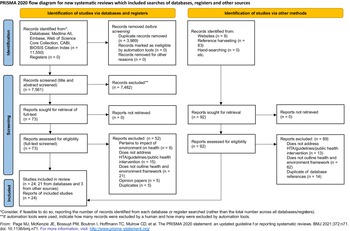
Figure 1. PRISMA flow diagram. Of 7,653 records identified, screening by title, abstract, and full text according to the eligibility criteria yielded 24 studies that were included in this review.
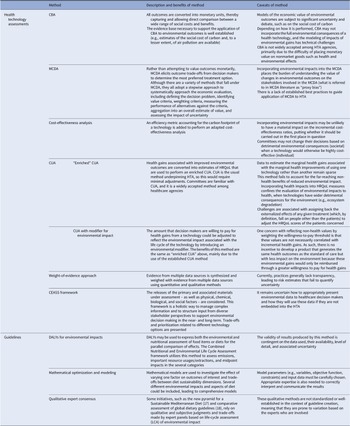
Table 1. Summary of methods used to integrate environmental considerations into HTAs and guidelines
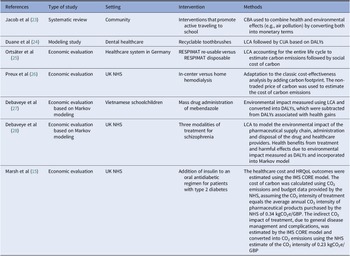
Table 2. Summary of case studies
- 19
- Cited by



Introduction
The detrimental impact of human activity on the environment – in terms of climate change, natural resource depletion, and biodiversity loss – is now undeniable and requires urgent intersectoral action (Reference Fears, Abdullah and Canales-Holzeis1). Environmental degradation and climate change threaten the foundations of good health, with direct and immediate consequences for population health and, hence, health systems (Reference Rocque, Beaudoin and Ndjaboue2). Worldwide, healthcare systems account for about 4–5 percent of global carbon emissions, which would make them the fifth largest country in terms of carbon emissions.
In January 2020, the National Health Service (NHS) in England was the world’s first national health system to commit to reaching net zero for directly controlled carbon emissions by 2040 (3). This was accompanied by a comprehensive plan to remove the 6.1 metric tons of CO2 equivalents (MtCO2e) required to reach net zero (3). Health systems have a variety of levers to reduce their carbon footprints, such as their supply chains, estates and facilities, pharmaceuticals and medical devices, care pathways, and staff and patients’ travel (Reference Ossebaard and Lachman4;Reference Drew, Christie, Tyedmers, Smith-Forrester and Rainham5). Healthcare decisions are often based on clinical guidelines and health technology assessments (HTAs) issued by the agencies, such as the National Institute for Health and Care Excellence (NICE) in England. Accounting for a broader range of outcomes beyond health, such as environmental impact, would allow decision makers to better maximize social welfare, assuming sustainability and environmental protection are valued by societies. This is a key step to ensure high-quality, cost-effective health care is consistently delivered across the country, while contributing to the sustainability and health of the ecosystem as a whole.
However, evidence is scant on how best to incorporate environmental considerations into clinical guidelines and HTAs (Reference Marsh, Ganz and Hsu6). The current methods and processes underpinning the development of guidelines and HTAs focus on maximizing health and prioritizing limited resources equitably and fairly. Efforts to formally include environmental considerations into guidelines have been largely restricted to dietary guidelines, acknowledging the heavy carbon footprint of our complex food systems (Reference Tuomisto7). Evidence of integration of environmental considerations into HTAs is also sparse, with a lack of consistency in methodological approaches (Reference Polisena, De Angelis, Kaunelis, Shaheen and Gutierrez-Ibarluzea8). Nonetheless, there is increasing public pressure and demand from healthcare stakeholders for environmental considerations to be formally incorporated into guidelines and HTAs (Reference Tanios, Wagner and Tony9).
In this context, this scoping review aims to
1. summarize the evidence currently available on frameworks and methods that enable inclusion of environmental considerations into guidelines and HTAs and
2. identify gaps in current knowledge that should be addressed by future research.
This scoping review is the first step of a larger initiative by NICE to develop processes and methods to integrate environmental sustainability into the development of its guidance products.
Methods
This scoping review was conducted according to the principles of the PRISMA-ScR Guidelines for scoping reviews (Reference Peters, Godfrey and Khalil10;Reference Peters, Marnie and Tricco11). The protocol for this scoping review was registered on the Open Science Framework (https://osf.io/4xvhs).
Inclusion criteria
Population
All types of study were eligible, including reviews, modeling studies, health economic evaluations, or case studies if they provided a basis for a framework or method.
Concept
Studies that described a conceptual framework, method, or approach used to integrate environmental and/or sustainability considerations into HTAs or the creation, revision, or recommendation of clinical practice or public health guidelines. Sustainability was defined as “meeting the needs of the present without compromising the ability of future generations to meet their own needs” following the United Nations definition (12). HTAs and guidelines on any clinical or public health areas were included.
Context
Studies conducted in healthcare or public health settings were included.
Exclusion criteria
The search was restricted by the date of publication from 2009 to present. The 2009 limit was based on studies included in a previous comparable review (Reference Polisena, De Angelis, Kaunelis, Shaheen and Gutierrez-Ibarluzea8), all bar one of which were published from 2009 onwards. Conference abstracts/papers, editorials, patents, or company profiles were excluded. Only articles in English were included.
Information sources
The bibliographic databases searched were Embase, MEDLINE ALL, Web of Science Core Collection, CABI via Web of Science, and BIOSIS Citation Index via Web of Science. All databases were searched on the 18th of July 2021. This search was supplemented by gray literature searching through the Web sites of key HTA or public health/environmental institutions. These sites were identified through the Canadian Agency for Drugs and Technologies in Health (CADTH) Grey Matters tool for gray literature searching (13) and are listed in Supplementary Table 1. In addition, three leading journals in the field of HTA were hand searched: Value in Health, International Journal of Technology Assessment in Health Care, and National Institute for Health Research Health Technology Assessment from January 2018 to August 2021. Forward and backward reference harvesting was done using Citation Chaser on relevant papers (Reference Polisena, De Angelis, Kaunelis, Shaheen and Gutierrez-Ibarluzea8;Reference Marsh, Ganz and Hsu14–Reference Pekarsky16).
Search strategy
The search strategy was developed based on a list of environmental impact terms, HTA/guideline terms, and health economics terms. The search was done by SY and peer reviewed by an information specialist, who also developed the search queries for all databases. Search was limited by date (1st January 2009 to 18th July 2021), language (English), and publication type (no editorials, letters, conference abstracts/conference papers). The full search strategy, including search queries for each database, is provided as Supplementary Material. The gray literature search was done by SY using search bars that were available on each Web site with combinations of search terms such as “environment/environmental,” “HTA/health technology assessment,” “sustainability,” “assessment,” and “guideline.”
Selection of sources of evidence
All references were deduplicated and screened independently by two reviewers (AA, ACPG, HM, MT, and SY) based on titles and abstracts and according to the eligibility criteria. No pilots were performed before screening. Full-text screening was then performed by ACPG and SY. Any discrepancies regarding which references to include were resolved by consensus or by consulting a third reviewer when a consensus could not be reached. The software EPPI-Reviewer 5 and Microsoft Excel were used for reference management and screening.
Data charting process
Data were extracted independently by two reviewers (ACPG and SY) for variables related to the article (title, author, year, setting, population), the type of study (review, modeling study, economic evaluation), the intervention/technology, the environmental impact assessment, and the method of environmental impact integration into the HTA or guideline. No pilots were performed before data extraction. Discrepancies between reviewers were resolved by consensus or by consulting a third reviewer when a consensus could not be reached. All data extracted from reports were summarized using tables.
Results
In total, 7,561 unique records were identified via databases (Fig. 1; a list of excluded studies at full-text screening is provided in Supplementary Table 2). Ninety-two records were identified via other methods. Screening by title, abstract, and full text according to the eligibility criteria yielded twenty-four studies to be included in this review (Supplementary Table 3).
Figure 1. PRISMA flow diagram. Of 7,653 records identified, screening by title, abstract, and full text according to the eligibility criteria yielded 24 studies that were included in this review.
Below, we discuss in detail the frameworks and methods identified, subdivided according to whether these apply to HTAs or guidelines, and review case studies in each case to illustrate the challenges of practical implementation. Table 1 summarizes the main methods described to support inclusion of environmental considerations into HTAs and guidelines.
Table 1. Summary of methods used to integrate environmental considerations into HTAs and guidelines
CBA, cost–benefit analysis; CEASS, comprehensive environmental assessment; CUA, cost-utility analysis; DALYs, disability-adjusted life-years; HRQoL, health-related quality of life; HTA, health technology assessment; LCA, life-cycle assessment; MCDA, multicriteria decision analysis.
Health technology assessments
A total of ten studies referred to integration of environmental considerations and sustainability into HTAs, of which seven were case studies and three were methodological papers. Overall, the studies typically adopted a two-stage approach. First, the environmental impact of a health technology was estimated using different methods, mainly life-cycle assessment (LCA). Second, the environmental effects were integrated into HTAs using several methods, such as “enriched” cost-utility analysis (CUA) (i.e., converting environmental impact into health-related quality of life (HRQoL) or disability-adjusted life-years (DALYs)), cost–benefit analysis (CBA) (based on monetization of environmental effects using, for instance, the social cost of carbon or non-traded cost of carbon), and multicriteria decision analysis (MCDA).
Methodological papers
Marsh et al. (Reference Marsh, Ganz and Hsu14) carried out the most comprehensive review of methods that support integration of environmental considerations into HTA. The authors argue the first challenge is to estimate the environmental impact of a health technology, which should consider the entire life cycle of that technology and its implications on resources used throughout the care pathway. This can be achieved using environmentally extended input–output analysis to estimate carbon emissions generated by each unit of output in a sector, but disaggregated data may not be available for each technology. LCA is a suitable option, but it may be infeasible due to lack of data or resources. The second challenge is to embed the environmental impact into HTAs, and the authors propose three methods, namely “enriched” CUA, CBA, and MCDA (Table 1).
Polisena et al. (Reference Polisena, De Angelis, Kaunelis, Shaheen and Gutierrez-Ibarluzea8) conducted a scoping review on frameworks and methods that could help CADTH develop its own methods and processes to incorporate environmental impact into HTAs. This review describes the frameworks proposed by Marsh et al. (Reference Marsh, Ganz and Hsu14) to integrate environmental impact into HTAs. In addition, it considers different approaches to measure the environmental impact of health technologies, such as the weight-of-evidence approach (Reference Springmann, Spajic and Clark19;Reference Hall, Belanger and Guiney20) and the comprehensive environmental assessment (CEASS) framework (Reference Linkov, Loney, Cormier, Satterstrom and Bridges21). Although the latter has the benefits of incorporating both quantitative and qualitative information and various stakeholder perspectives into the analysis, as well as presenting the trade-offs and prioritization related to different technology options, its usability is limited by not embedding the environmental impact into the economic model underpinning the HTA.
Hensher (Reference Powers, Dana and Gillespie22) explored the theoretical and practical considerations involved in incorporating environmental sustainability directly into the economic evaluation of health technologies by drawing on concepts related to ecological economics and describing how multiple techniques can be used to elicit the value of the environmental impacts of health care.
Case studies
The seven case studies are summarized in Table 2, and the key implications of their findings are described briefly in this section.
Table 2. Summary of case studies
CBA, cost–benefit analysis; CUA, cost-utility analysis; DALYs, disability-adjusted life-years; HRQoL, health-related quality of life; kgCO2e/GBP, kilograms of CO2 equivalents per British pound sterling; LCA, life-cycle analysis; NHS, National Health Service.
Overall, the case studies demonstrated LCA can be successfully used to estimate the environmental impact of technologies (Reference Duane, Ashley and Saget24;Reference Jacob, Chattopadhyay and Reynolds28), including both direct effects (i.e., raw materials consumed, as well as waste and emissions generated during the manufacturing, distribution, and use of the technology) and indirect effects (i.e., due to the impact of the technology’s health outcomes, which will impact a patient’s need for other treatments and services, each of which generates its own environmental impacts) (Reference Marsh, Ganz, Nørtoft, Lund and Graff-Zivin15). In fact, Debaveye et al. (Reference Jacob, Chattopadhyay and Reynolds28) suggested indirect effects may be more significant than direct effects.
On the other hand, case studies also highlighted some challenges, such as (i) lack of data on environmental impact in a form that could be incorporated into economic models other than for CO2 emissions and (ii) CO2 emissions data not being available at a sufficiently disaggregated level to isolate the impact of individual technologies (Reference Marsh, Ganz, Nørtoft, Lund and Graff-Zivin15). This lack of data underpins why partial rather than full LCA was commonly adopted.
Some case studies (Reference Duane, Ashley and Saget24–Reference de Preux and Rizmie26;Reference Jacob, Chattopadhyay and Reynolds28) demonstrated that the technology or treatment pathway with the least environmental impact (i.e., the more sustainable option) was also the most cost-effective from a health perspective. This means discounting the health loss caused by the negative effects on the environment had no material impact on the decision to recommend or not a technology based on the conventional CUA. Considering the hefty resources of estimating environmental impact of health technologies, routine environmental impact assessment may not be warranted. Rather, it may be reserved for technologies expected to have a substantially negative environmental impact. However, a low relative reduction in environmental impact may translate into a large absolute impact if a technology is widely used, as is in the case of inhalers (Reference Ortsäter, Borgström, Baldwin and Miltenburger25). On the other hand, studies emphasized the current lack of clarity over how committees and agencies might handle the trade-off between environmental and health effects, particularly when considering detrimental environmental consequences (largely a societal outcome) when a technology would otherwise be highly cost-effective (mainly an individual outcome) (Reference Duane, Ashley and Saget24–Reference de Preux and Rizmie26;Reference Jacob, Chattopadhyay and Reynolds28).
Guidelines
There was no study specifically on a framework or method to incorporate environmental considerations into clinical and public health guidelines. However, there were some case studies and reviews that discussed how certain elements of sustainability could be considered in different aspects of health care. A significant proportion of these dealt with dietary guidelines. Although food-based dietary guidelines are beyond the scope of NICE, the frameworks and methods developed and applied by those guidelines may be transferrable to clinical and public health guidelines, even if some adjustments are required.
Health care
Systematic reviews carried out by Seifert et al. (Reference Debaveye, Gonzalez Torres and De Smedt29) and Reynier et al. (Reference Debaveye, De Smedt, Heirman, Kavanagh and Dewulf30) demonstrated how LCA can be used to estimate the carbon footprint of hospitals, particularly regarding operating room management (Reference Seifert, Koep, Wolf and Guenther31–Reference Sherman, Raibley and Eckelman33). However, none of the papers included in those reviews addressed how to integrate environmental considerations into guidelines. They focused instead on how environmental impact should be considered in decision making at the hospital or individual level in a qualitative and subjective manner, instead of proposing models or methods to achieve this in an objective and systematic way. Furthermore, those reviews highlighted that reliance on carbon footprint as the sole measure of environmental impact of health care was oversimplistic and failed to account for the far-reaching consequences on ecosystems.
NICE also published a report on the environmental impact of implementing their guideline on medicines optimization (Reference McGain, Story, Lim and McAlister34), which estimated the total greenhouse gas (GHG) emissions that could be avoided by medicines optimization both through direct (e.g., manufacturing and supply of pharmaceuticals) and indirect effects (e.g., reducing hospital stays by avoiding adverse drug reactions). This report illustrated how changing healthcare practice as advised by guidelines could have co-benefits for the environment, even if this was a supplementary analysis not formally embedded into guideline development (which was based on conventional considerations of health benefits and costs). NICE has also produced a decision aid for patients and clinicians that includes environmental considerations in decision making about inhalers for asthma, but to date this has not been incorporated into guidance (Reference Vozzola, Overcash and Griffing35).
Food and dietary guidelines
A total of ten articles related to food and dietary guidelines were included in this review, which broadly addressed how to measure the environmental impact of foods and diet and how to integrate that information into dietary guidelines.
Measuring the sustainability and/or environmental impact of foods and diet
Systematic reviews carried out by Jones et al. (36) and by Eme et al. (37) found sustainability of diets was most commonly assessed using LCA to estimate GHG emissions. Other indicators related to biodiversity, land and water resources, and ecosystem health were seldom considered. Two case studies by Huseinovic et al. (Reference Jones, Hoey and Blesh38) and Bozeman et al. (Reference Eme, Douwes, Kim, Foliaki and Burlingame39) illustrated the successful implementation of LCA methods to estimate the benefits of dietary interventions on health and environmental outcomes. Importantly, both studies relied on partial LCA, which did not account for the full life cycle of food systems.
Integrating environmental and sustainability considerations into guidelines
Although LCA methods allow estimating the environmental impact of diets, the challenge is to take that impact into account when making decisions about food-based dietary guidelines. There are broadly two quantitative methods and one qualitative method. First, environmental and nutritional impacts can be converted into utility measures, such as DALYs, which can be traded directly, as shown by Stylianou et al. (Reference Huseinovic, Ohlin and Winkvist40). Second, mathematical simulation-optimization models can help find the ideal trade-off between health, nutrition, and environment when given a set of rules and constraints. Examples of how these models strike the balance between health and environment when producing dietary guidelines are provided by Gazan et al. (Reference Bozeman, Springfield and Theis41), Wilson et al. (Reference Stylianou, Heller and Fulgoni42), and Brink et al. (Reference Gazan, Brouzes and Vieux43). These optimization models may be transferrable to health care by adjusting the model parameters. Third, it is possible to make qualitative judgments on the relative value of environmental and health impacts by expert panels, with variable degrees of involvement by the general public. For example, Serra-Majem et al. (Reference Wilson, Cleghorn, Cobiac, Mizdrak and Nghiem17) described the addition of sustainability dimension at the base of a new pyramid for a Sustainable Mediterranean Diet. Springmann et al. (Reference Brink, van Rossum and Postma-Smeets18) modeled the health and environmental impact of adoption of eighty-five national food-based dietary guidelines and the methods used could be applied, albeit qualitatively, by healthcare guideline developers.
Discussion
This scoping review demonstrates there is some evidence on how to integrate environmental considerations into HTAs, with examples of successful implementation of methods such as MCDA, CBA, or “enriched” CUA. However, all these methods have significant shortcomings and require further improvements prior to widespread adoption by HTA agencies. On the other hand, evidence is limited on how to integrate environmental considerations into clinical and public health guidelines and no framework was identified by this review. Food and dietary guidelines have been applying different methods, such as utility-based models or simulation-optimization modeling, to inform decision making for a longer time. These methods may be transferrable to clinical and public health guidelines, albeit with modification. In recent years, evidence has been rapidly accruing on how to measure environmental impacts of health care in general and specific technologies, mainly based on LCA methods like those used by food and dietary models. Despite this growth in interest and work that has been done, further research is warranted to develop methods for incorporating environmental considerations into decision making in health care and prioritizing technologies that are most likely to benefit from the required investment of resources.
This scoping review has important implications for NICE and other agencies worldwide dedicated to guideline production and HTA. Despite general consensus about the importance of and pressing need to incorporate environmental and sustainability considerations into guidelines and HTAs, there are still substantial challenges to be overcome before this can be accomplished. These challenges can be conceptualized at two levels: (i) measurement of the environmental impact of health technologies and (ii) embedding environmental impacts into guidelines and HTAs.
Although detailed analysis of the methods underpinning environmental impact assessment is beyond the scope of this review, it is a vital consideration as it is the first step when discussing the inclusion of environmental impact in guidelines and HTAs. This review identified different methods, but the most used and widely accepted is LCA. A holistic evaluation of the environmental impact of a health technology requires very detailed data, which may not be available or may be impractical to gather. This data requirement, in particular, implies that LCA is typically costly and time- and resource-intensive, even though its comprehensive cradle-to-grave approach justifies its use in a variety of healthcare settings. In this context, it is perhaps unsurprising that many studies performed only partial LCAs, which accounted for some but not the entire life cycle of a technology for practical reasons or due to lack of data. In addition, studies have emphasized the lack of data on environmental indicators other than carbon emissions and some authors have proposed frameworks to fully evaluate the environmental impact of pharmaceuticals. These enable including less traditional metrics, such as impact on water resources, livestock infections, antimicrobial resistance, and emergence of new diseases, thus extending the scope of environmental impacts that can be added to economic models beyond carbon emissions (Reference Tarazona, Escher, Giltrow, Sumpter and Knacker44;Reference Coutu, Rossi, Barry and Chèvre45). Even when data are available, they are seldom available with enough granularity to estimate the environmental impact of specific technologies. For instance, there are several modeling studies on aggregate carbon footprints of healthcare bundles or services, and the NHS estimated the average annual CO2 intensity of pharmaceutical products as 0.34 kgCO2e/GBP (kilograms of CO2 per British pound sterling). However, data are scant or lacking for the carbon footprint of specific health technologies, especially if they are new and have only been used in a research context, as is often the case with HTAs. To address this deficiency, it is arguable whether it should be mandatory for clinical trials investigating new technologies to conduct environmental impact assessment alongside and report both health and environmental outcomes. Such an approach would, however, need to be proportionate, such that it did not unduly discourage pharmaceutical companies from developing new technologies that could improve and extend the lives of many people. Furthermore, compiling and analyzing all the data required for a full LCA may be infeasible within the current timeframe of HTAs. A full LCA approach may therefore be incompatible with NICE’s core principle of ensuring timely access to new technologies in the NHS. This review has, thus, highlighted the need for further research to support the measurement of the environmental impact of health technologies. Systematic and thorough methods together with reliable and valid data are needed to measure the environmental impact of health technologies in a timely and practical manner.
Once the environmental impact assessment is completed, it will feed into HTA and guideline development. This second step brings additional challenges (Reference Nichols, Maynard, Goodman and Richardson46). The current methods underpinning HTAs are not well suited to integrate environmental considerations. Although several methods have been proposed, such as “enriched” CUA, CBA, or MCDA, all of them have significant shortcomings. For instance, environmental consequences are often far-reaching and hence utility measures centered on individual health (e.g., DALYS or HRQoL) do not adequately capture the full extent of the environmental benefits and/or harms. Although monetization of environmental impact could be an option, monetization of health remains a controversial area and it is unclear whether HTA agencies and societies and public opinion, in general, would be prepared to accept this as an approach. There is even greater uncertainty on how best to incorporate environmental considerations into clinical and public health guidelines. Although several methods have been developed for food-based dietary guidelines, their adaptation to clinical and public health guidelines may not be straightforward, not least due to the lack of availability of relevant data. Food-based dietary guidelines are often able to advance both health and environmental sustainability initiatives, rather than having to balance gains in one aspect for losses in the other as is typically the case in HTAs and clinical practice guidelines. For instance, a review of public health interventions to increase urban green space found that all thirty-eight studies measured only health or environmental impacts but not both (Reference Hunter, Cleland and Cleary47). This means a direct comparison and discussion of the trade-off between health and environmental outcomes is impossible at this stage. Future studies, either observational or experimental, should consider both health and environmental outcomes to inform decision making in healthcare and public health policy, while preserving key ethical principles at the core of healthcare systems, such as equity.
Finally, the consequences of including environmental impact in guidelines and HTAs remain uncertain. First, it is unclear whether and when it may have a material impact above and beyond incremental cost-effectiveness ratios (ICERs) or other conventional health economics metrics, and hence on recommendations. Some case studies showed that considering environmental impact as a modifier in economic models had barely any effect on ICERs, thus calling into question the rationale for pursuing it (Reference Duane, Ashley and Saget24–Reference de Preux and Rizmie26;Reference Jacob, Chattopadhyay and Reynolds28). It is possible too that the impact on ICERs is strongly dependent on the underlying methods used, which is problematic considering that there is currently no consensually accepted method. The possibility that environmental impact considerations may not materially change decisions is particularly important in view of the heavy resource requirement to measure environmental consequences. Given these concerns, incorporating environmental impact into guidelines or HTAs may be best reserved for technologies that are likely to have a significant environmental impact. Ideally, all technologies should be screened for their potential environmental impact. However, the criteria that should be used to identify technologies that warrant a comprehensive environmental impact evaluation remain unclear (Reference Polisena, De Angelis, Kaunelis, Shaheen and Gutierrez-Ibarluzea8). In addition, the overall impact of a health technology may still be substantial, despite a small impact at the individual patient level, if the technology is very commonly used in the population. Second, the trade-off between health (largely an individual outcome) and environment protection (largely a collective outcome) and willingness to pay for environmental improvement and/or avoidance of degradation are ethical issues that lie outside the remit of HTA and guideline committees and should be resolved by society or their elected representatives. As methods supporting environmental impact assessment improve and societal values evolve over time, discussions on whether and how to integrate environmental considerations into HTA and guidelines should be revisited.
Limitations
Although this scoping review was based on a comprehensive literature search, including gray literature search, it is still possible that some papers may have been missed. As it relied on published literature, publication bias cannot be excluded. Given the English language limitation, publications in other languages without English translations may have been missed. The gray literature search was focused and hence may have missed some relevant records.
Conclusion
In principle, there is broad support among policy makers, academics, and society for taking into account the environmental and sustainability implications of healthcare interventions and technologies in the development of HTAs and guidelines (48–Reference Buse, Tomson and Kuruvilla50). In practice, there are many questions yet to be answered before this can be implemented widely. This review showed frameworks to support incorporation of environmental considerations into HTA and clinical and public health guidelines are lacking, and methods are in early stages of development and have significant technical limitations in terms of data and analytical approaches. Besides these methodological challenges, there are important ethical and political issues to be addressed at the societal level regarding trade-offs between health and environmental outcomes and monetary resource allocation. Further research is warranted to develop robust methods to incorporate environmental considerations into HTA and guideline development as well as to identify their place in different value systems across societies worldwide.
Supplementary material
To view supplementary material for this article, please visi https://doi.org/10.1017/S0266462322003282.
Acknowledgments
The views expressed in this paper do not necessarily reflect the views of NICE as an organization.
Conflict of interest
The authors declare none.