Major depression, a common disabling clinical condition, affects 21/1000 (point prevalence) 16 to 65-year-olds in the United Kingdom. The lifetime prevalence in individuals with diabetes is 24 percent (Reference Goldney, Phillips and Fisher1), three times higher than the general population. Clinically relevant depression affects around 30 percent of patients with diabetes and depression (Reference Anderson, Freedland and Clouse2). People with diabetes plus depression are less likely to be physically and socially active, effectively communicate with healthcare practitioners or comply with diet and treatment than people with diabetes alone. These factors can lead to worse long term complications and higher mortality (Reference Von Korff, Katon and Lin3;Reference Piette, Heisler and Wagner4). Problems with detection and treatment of depression are compounded by long term conditions and patients and healthcare professionals normalize symptoms of depression and distress (Reference Coventry, Hays and Dickens5).
In the UK, depression is ordinarily managed in primary care. UK government policy promotes increased access to mental health care through commissioning and provision of health and social care in primary care settings, supported by the Improving Access to Psychological Therapies (IAPT) initiative and cross governmental strategy to improve the balance between mental and physical health care. Policy initiatives to improve access to and quality of low-intensity interventions such as talking therapies focused on innovations in service delivery in primary care.
Management in primary care can support patients and practitioners in avoiding talking about depression (Reference Coventry, Hays and Dickens5;Reference Ridd, Shaw and Salisabury6). This is reinforced by highly managed and time-limited GP consultations in UK primary care (Reference Chew-Graham and Hogg7). Mental health services are separated from general practice, with poor access to psychological services, hindering integrated, effective treatment (Reference Telford, Hutchinson, Jones, Rix and Howe8).
Effective care for depression in patients with diabetes requires changes in the attitudes, beliefs and behaviors of healthcare practitioners, with care models based on educational and organizational changes in primary care (Reference Gilbody, Whitty, Grimshaw and Thomas9). Collaborative care models that include psychological interventions may be effective and cost effective in people with depression or long term conditions when healthcare systems are barriers to effective treatments (Reference Katon, Schoenbaum and Fan10–Reference Katon, Lin and Von Korff12). Qualitative research and a systematic review of psychological interventions to manage depression in diabetes indicated the need for new approaches to overcome the barriers to using evidence based interventions in long term conditions (Reference Coventry, Hays and Dickens5;Reference Harkness, Macdonald and Valderas13).
However, decision makers need information about the relative costs, benefits and cost-effectiveness to inform policy and practice changes to implement new interventions. This study reports a focused systematic review of the cost-effectiveness of psychological interventions in people with depression and diabetes. The aims were to: (i) Identify and review current economic evidence of interventions for people with diabetes and co-morbid clinically relevant depression. (ii) Identify the level of robustness or uncertainty of economic evidence about the management of co-morbid depression (with diabetes). (iii) Inform the design of prospective studies to support evidence based policy and practice and identify key data to collect.
METHODS
Search Strategy
The search strategy (Supplementary Table 1, which can be viewed online at http://dx.doi.org/10.1017/S0266462313000445, shows the final search used in Medline) combined economic search terms (Reference Katon, Unutzer and Fan14), with clinical terms from a previous review (Reference Harkness, Macdonald and Valderas13) and validated by checking whether it identified publications known to the research team. Electronic searches were limited to January 2000 to May 2012. Older studies may not reflect current service organization, provision or funding which have changed substantially in recent years. The search strategy was adapted for each of the electronic databases searched, using the Ovid interface: Medline, Embase, PsycINFO, CINAHL, and NHS Economic Evaluation Database (NHS EED) databases.
Inclusion Criteria
Studies were included if they were published in English, reported full economic evaluations with a synthesis of net costs and outcomes in adults, compared two or more interventions to manage depression in people with diabetes. Policy papers, cost of treatment or burden of illness studies, letters, editorials, book reviews and poster presentations were excluded.
Data extraction and Assessment
Two authors (FJ and LD) screened titles and abstracts and differences were resolved by discussion. Full papers were obtained for titles and abstracts that met the inclusion criteria or were uncertain. Full papers were assessed for inclusion in the review. Data were extracted and critically assessed on a standardized form by FJ and LD, using NHS EED guidelines (14) and descriptively summarized using narrative tables.
RESULTS
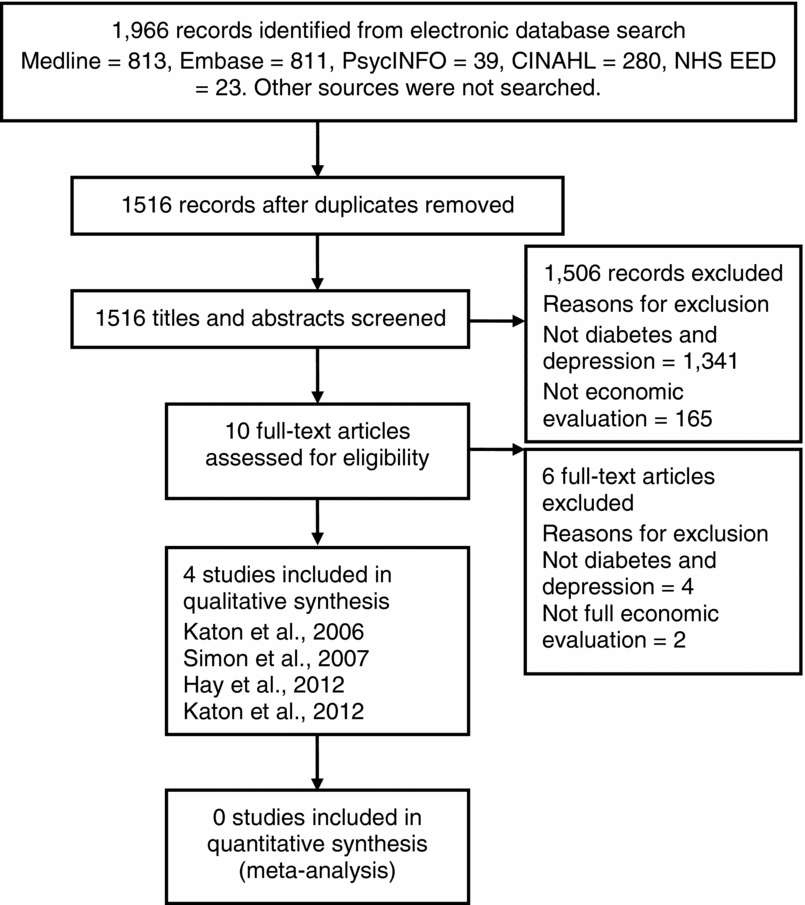
Figure 1 summarizes the number of studies retrieved and reasons for exclusion. Four economic evaluations alongside or integrated with randomized controlled trials (RCT) were included (15–18) and are summarized in Table 1 (and Supplementary Table 2, which can be viewed online at http://dx.doi.org/10.1017/S0266462313000445,). None of the four studies reported all the detail about participants and methods required for the summary tables or critical appraisal. Previously published reports of the evaluations were used to identify this information.

Figure 1. Studies identified and retrieved published between 2000 and May 2012.
Table 1. Study Designs for Evaluations of Interventions for Diabetes and Depression

Target Population and Participants
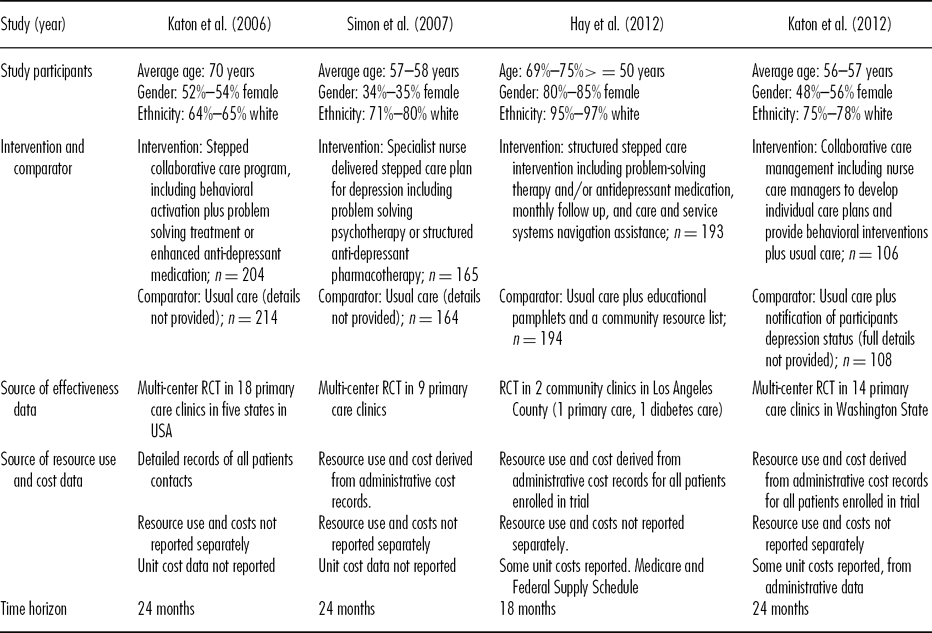
All studies were conducted in the United States and the target population was people with major depression and diabetes (15–17) and/or coronary heart disease (CHD) (Reference Katon, Russo and Lin18). In one study (Reference Katon, Unutzer and Fan15) the target population was elderly people (60 years or more) with major depression. For this study, a sub-group of participants included in the original study (Reference Unützer, Katon and Williams19) who also had diabetes were analyzed.
In one study, the majority of participants were predominantly Hispanic people. The authors noted this group were at higher risk of diabetes and co-morbid diabetes and depression than non Hispanic white people (Reference Hay, Katon and Ell17). In one study 23 percent to 30 percent of participants had coronary heart disease (CHD) (Reference Simon, Katon and Elizabeth18), whilst 82 percent to 89 percent had diabetes with or without CHD. This study did not present results separately for diabetes and CHD. There appeared to be differences in demographic characteristics between the usual care and intervention groups in all but one of the studies (Reference Hay, Katon and Ell16–Reference Simon, Katon and Elizabeth18).
Interventions
All studies evaluated collaborative care to help participants manage depression in primary care. They included a care planning process of stepped depression management tailored to individual needs, care manager, psychological therapy and/or antidepressant therapy. One study (Reference Katon, Russo and Lin18) actively targeted diabetes (or CHD) in the care plans. Care managers delivered psychological therapy and were trained/specialist nurses, incorporated into the primary care team. They were supervised by psychiatrists and/or primary care physicians to monitor progress and adjust treatment plans according to treatment response. Care managers contacted participants two to three times per month initially to monitor and discuss progress.
Usual care was not described in any of the papers.
Costs and Outcomes
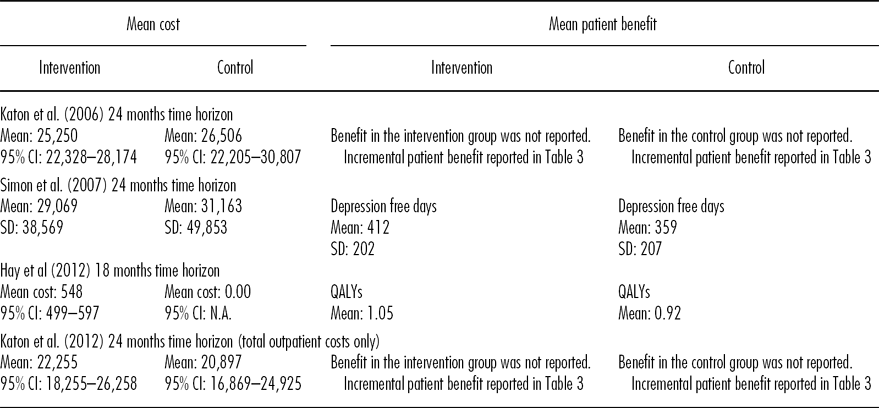
One study reported a cost-effectiveness analysis (Reference Simon, Katon and Elizabeth16) whilst cost-utility analyses were presented in the other three evaluations (Reference Katon, Unutzer and Fan15;Reference Hay, Katon and Ell17;Reference Katon, Russo and Lin18). All but one of the studies (Reference Hay, Katon and Ell17) used a time horizon of 24 months (15;Reference Hay, Katon and Ell16;Reference Simon, Katon and Elizabeth18), but costs and outcomes were not discounted in any of the analyses. Three studies reported a payor perspective (Reference Hay, Katon and Ell16–Reference Simon, Katon and Elizabeth18) and one reported a societal viewpoint for the analyses (Reference Katon, Unutzer and Fan15;19). Tables 2 and 3 summarize the costs and outcomes. Costs are adjusted to a single price year (USD 2011), using a medical care services index (Reference Payne, McAllister and Davies20). Total costs of the intervention group were USD22,250 to USD29,100 over 24 months, whilst total costs of the comparator were USD21,900 to USD31,200 (15; 16; Reference Katon, Russo and Lin18). One study (Reference Hay, Katon and Ell17) did not report the total costs of care. The authors found no statistically significant differences in the change in costs from 6 months prebaseline to the 18 month follow-up. On this basis they assumed that the only cost attributable to the intervention was the cost of providing the intervention so only included the additional costs of the intervention with the costs of the comparator assigned as zero (Reference Hay, Katon and Ell17).
Table 2. Total Costs (USD, 2011) and Patient Benefit

Table 3. Net Costs (USD, 2011), Outcomes, and Incremental Cost-Effectiveness Ratios

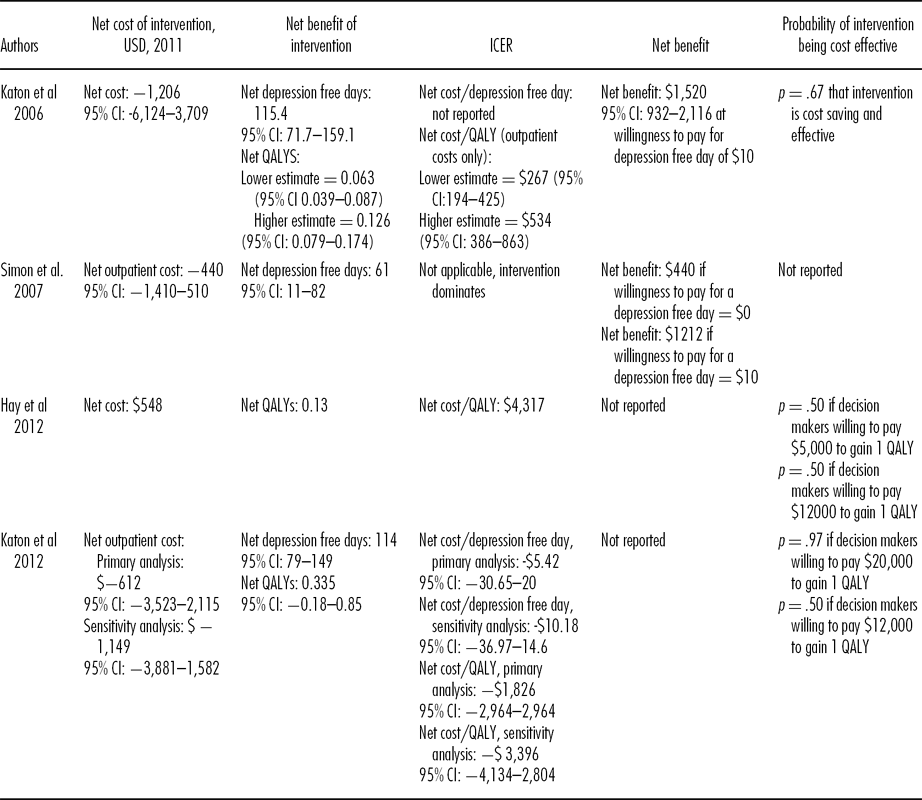
Three studies reported net savings (USD440-USD1206) for the intervention (Reference Katon, Unutzer and Fan15;Reference Katon, Russo and Lin16;Reference Simon, Katon and Elizabeth18). The 95 percent confidence intervals (CIs) all cross zero, suggesting no statistically significant difference in costs between intervention and control groups. One study reported a net cost (USD 548; 17).
Different measures were used to assess the impact of care. Outcomes were reported for the intervention and control group in two studies. These were depression free days (412 depression free days, intervention vs. 359 depression free days control; 16) and QALYs (1.05 intervention vs. 0.92 control; 17). Three studies reported net QALYs (0.063 to 0.335 QALYs gained), and two studies reported monetary measures of net benefit (USD 1212 to USD 1520).
Table 3 reports the net costs, outcomes and incremental cost-effectiveness ratio (ICER) of the interventions evaluated. These indicate less variation in the data.
Critical Assessment
Risk of bias
Double blinding treatment providers and patients was not feasible and all studies used blinded assessments, data entry and analysis. Whether this was successful was not reported.
Individual participants were randomized, increasing risk of contaminating practice and treatment between allocation groups. Sample size estimates were reported for effectiveness, not economic measures, and excluded cluster effects. Combined with substantial variance in the economic measures, it is unclear if sample sizes were sufficient to accurately estimate the ICER and uncertainty.
One study did not adjust for imbalances in participant baseline characteristics (Reference Katon, Unutzer and Fan15). Three studies did adjust for baseline characteristics (Reference Hay, Katon and Ell16–Reference Simon, Katon and Elizabeth18), but did not report a-priori identification of characteristics that influence health status or costs. One study included participants with CHD and no diabetes, but did not report results separately (Reference Katon, Russo and Lin18). It is not reported whether there were differences in the interventions’ cost-effectiveness or whether people with depression and diabetes are comparable to those with depression and CHD.
Only two studies accounted for missing data (Reference Katon, Unutzer and Fan15;Reference Hay, Katon and Ell17). Insufficient information was reported to assess the assumptions used and the robustness of the results.
Choice of Comparator
The comparator in all studies was usual care, but what this comprised was not described. The relevance of alternative comparators was not discussed. Differences in usual care between studies may explain some of the variation in the costs and outcomes reported.
Validity of Benefit and Cost Estimates
Three studies reported depression-free days and net monetary values of depression-free days (primary analysis), which exclude adverse events or broader health benefits of the intervention (Reference Katon, Unutzer and Fan15;Reference Katon, Russo and Lin16;Reference Simon, Katon and Elizabeth18). Different willingness-to-pay values to gain a depression-free day, were used, so are not directly comparable.
One study used the SF-12 and SF-6D tariffs to estimate QALYs (Reference Hay, Katon and Ell17). The SF-12 does not include all items for the SF-6D, reducing the reliability and validity of the estimated results. Two studies estimated QALYs by mapping published data onto net depression-free days (Reference Katon, Unutzer and Fan15), or imputing from diabetes markers (Reference Katon, Russo and Lin18). This excludes adverse events, reduces the robustness of the QALY estimate and reduces comparability with other evaluations.
Direct nonmedical costs were not reported. Insufficient detail was given to assess the impact of this on cost-effectiveness estimates. Usual care was not described, so it is not clear whether the range of costs included is appropriate. Total costs in one study only included outpatient costs (Reference Katon, Russo and Lin18). One study only reported the net additional costs of the intervention (Reference Hay, Katon and Ell17) excluding costs of other services to manage depression or diabetes. There is insufficient information about the method of identifying and attributing service use to assess the validity of this approach.
Length of follow-up ranged from 18–24 months. No study extrapolated results over patient lifetimes, which is important for diabetes and depression. All studies reported cost-effectiveness acceptability analyses and/or net benefit analyses but did not adequately report the methods or the relevance of the range of willingness to pay thresholds used.
Transferability of Results
None of the studies used sensitivity analysis to explore the transferability of the results to other settings or countries. All studies were conducted in the United States, and one person (Katon) was an author on all four. This may indicate all studies were based in similar healthcare systems and evaluated very similar interventions. If so, generalizability of the intervention and results to other settings/countries, where health care differs may be limited.
All studies were primary care based, using systematic screening to identify eligible patients, which may not be feasible elsewhere. It may also change the service setting, affecting the cost-effectiveness of the intervention. Information about adherence with treatment protocols and therapeutic alliances between healthcare professional and patients was not reported and can influence the transferability of results between settings/countries.
Whether the study samples were representative of the study populations or other populations was unclear. Most participants in one study (Reference Simon, Katon and Elizabeth16) had Type 2 diabetes (96 percent). Whether participants had Type 1 or Type 2 diabetes was not reported in three studies. The proportion of patients who screened positive for depression and were randomized ranged from 19 percent to 74 percent. The reasons for this variation were unclear.
No study reported unit costs, costs and resource use for all cost items, limiting transferability between settings/countries.
DISCUSSION
Implications for Policy and Practice
This study reports a systematic review of full economic evaluations of interventions to manage depression in people with diabetes. Four studies met inclusion criteria, indicating collaborative care including psychological therapy may be cost-effective.
There were limitations with this review and the studies assessed. This review was focused rather than comprehensive. No hand searches or searches of the grey literature were conducted, meaning key studies may have been missed. All the studies included in the review evaluated models of collaborative care. Collaborative care was not an objective of the review and not explicitly included as a search term in the search strategy. However, the search strategy did include terms relating to psychological or behavioral interventions, which were key elements of the collaborative care approaches. Clinical experts in the research team did not identify any additional evaluations that were not identified in the electronic searches. The search strategy did not explicitly include CHD as a search term, whereas 1 study included participants with depression and diabetes and/or CHD. It is not possible to assess from this study whether the results for the two groups are comparable.
Assessment of the internal validity and robustness of the four studies was limited by the information reported about the study design and methods. All the studies appeared to have limitations in study design that increased the risk of selection and measurement bias. Issues in the measurement of costs and health benefit limit the validity of the estimates.
The generalizability of the results to other settings and countries may be limited. Key issues to consider are first, whether the health systems and organization of care in the studies, (e.g., funding and patterns of service use) are typical of those found in other parts of the United States or other countries. Second, whether the intervention requires highly trained/specialist staff to deliver it to achieve the same level of adherence, therapeutic alliance and effectiveness, and the feasibility and acceptability of this in other settings/countries. Third, whether the predominantly older participant samples are representative of the populations treated in other settings in terms of age, type of diabetes (1 or 2) and main setting of care. A related point is whether screening will be needed to identify patients and whether this is feasible.
Implications for Research
The sample sizes reported may not be sufficient to accurately represent the incremental cost-effectiveness of the intervention or identify important differences in costs or health benefit. Evidence from clinical research demonstrates that small studies are associated with over-estimates of effects. Larger, well controlled, prospective evaluations are needed to inform evidence based policy and practice about cost-effectiveness. The evaluations also need to take into account clustering effects where experience from providing the intervention may influence the type and quantity of care in the comparison group. The studies reviewed provide information to estimate the sample sizes for economic endpoints such as the net benefit statistic.
Work is needed to identify the relevant range of service use. For example, costs of participant and family time (as inputs to the production of care), purchase of care from private providers and the costs of community and social care services are relevant in some settings.
The measure of patient benefit in future evaluations should reflect the impact of care on participants overall health. This is particularly important if psychological therapies indirectly affect adherence and the effectiveness of diabetes care. Qualitative and quantitative studies are needed to identify, from the patient's perspective, key attributes of health and estimate the relative utility or value of these. The extent to which QALYs and measures to estimate QALYs are relevant to these patient groups should be explored (Reference Payne, McAllister and Davies21).
Evaluations in broader samples of participants are needed. The majority of participants in the studies were older, white women. Screening and selection of participants means they are not representative of all people with diabetes and depression. Future evaluations require procedures to minimize the risk of recruitment bias. Additionally, collecting demographic characteristics of patients who do not participate could facilitate analytic controls for this problem. For example, survey analysis methods to weight participants’ data according to over and under-represented characteristics may help to reduce the impact of recruitment biases in the study sample.
Future evaluations should minimize missing observations and data in participants who complete scheduled follow-up as well as encourage complete follow-up. Where missing observations and censored data occur analytic approaches to impute the missing data are required. These should be selected to minimize biases and adequately represent uncertainty.
Economic models are needed to extrapolate the results to other settings and populations, and longer time horizons as well as provide the decision maker with the information required for evidence based policy and practice. This requires more detailed reporting of the methods, assumptions and results of prospective evaluations to support formal synthesis of economic evidence and development and analysis of economic models.
CONCLUSION
A recent Cochrane review concluded that collaborative care is an effective approach to the management of depression and anxiety (Reference Archer, Bower and Gilbody22) and there is evidence to support its use in managing co-morbid depression and diabetes (Reference McGregor, Lin and Katon23). Economic evidence suggests that from a U.S. payers perspective, collaborative care to manage depression in people with diabetes is associated with gains in health benefit and may be cost saving. However, as discussed above, there are several issues that limit the relevance of the results to alternative settings, timeframes, and populations. These include differences in health systems and organization of care between settings/countries, the feasibility and acceptability of the intervention in other settings/countries, the selected samples of participants may not be representative of routine practice. Additionally, there is substantial uncertainty about the robustness of the evidence. Variation in results in the studies could reflect differences in: the perspective and range of costs included; the availability and organization of care for long-term conditions and depression between settings and populations; the methods used to estimate costs and benefits; and the characteristics of the study samples recruited.
SUPPLEMENTARY MATERIAL
Supplementary Tables 1 and 2 can be found at: http://dx.doi.org/10.1017/S0266462313000445
CONTACT INFORMATION
Farheen Jeeva, MSc, Research Associate (to May 2013), Collaboration for Leadership in Applied Health Research and Care for Greater Manchester, Institute of Population Health and Manchester Academic Health Sciences Centre, University of Manchester, Manchester, UK
Christopher Dickens, MB, BS, PhD, Professor, University of Exeter Medical School and Peninsula Collaboration for Leadership in Applied Health Research and Care (PenCLAHRC), University of Exeter, Exeter, UK
Peter Coventry, PhD, Research Fellow, Collaboration for Leadership in Applied Health Research and Care for Greater Manchester, Institute of Population Health and Manchester Academic Health Sciences Centre, University of Manchester, Manchester, UK
Christine Bundy, PhD, Lecturer, Institute of Inflammation and Repair and Manchester Academic Health Sciences Centre, University of Manchester, Manchester, UK
Linda Davies, MSc, (linda.davies@manchester.ac.uk) Professor, Collaboration for Leadership in Applied Health Research and Care for Greater Manchester, Institute of Population Health and Manchester Academic Health Sciences Centre, University of Manchester, Manchester, UK
CONFLICTS OF INTEREST
All authors report a grant to their institution from NIHR Collaboration for Leadership in Applied Health Research and Care for Greater Manchester. Christopher Dickens also reports support for travel and support in kind from the same source.