Dear Dr Mäkelä,
In our article “Romiplostim and eltrombopag for immune thrombocytopenia: methods for indirect comparison” published in the Journal (Reference Cooper, Fitzgerald, Dillingham, Helme and Akehurst1), we presented an indirect comparison of the effectiveness of eltrombopag and romiplostim in raising platelet counts in patients with immune thrombocytopenia (ITP). Indirect comparison analyses are recommended by the National Institute for Health and Care Excellence (NICE) in cases where randomized head-to-head studies do not exist, and were used by NICE in their guidance for the eltrombopag Single Technology Appraisal submission (2).
Following publication of our study, updated data from the eltrombopag RAISE study were included in the evidence package to support the NICE final guidance regarding eltrombopag for the treatment of ITP (2;3). These updated data included fourteen additional patients receiving eltrombopag and one additional patient receiving placebo assessed as having an overall platelet response, and six additional eltrombopag patients and no additional placebo patients assessed as having a durable platelet response (Table 1). We would like to describe the relevance of our original analyses in light of these new data, such that readers of International Journal of Technology Assessment in Health Care are aware of the full range of evidence available for informing health policy decisions on the use of eltrombopag and romiplostim.
Table 1. Comparison of Platelet Response Rates between Eltrombopag and Romiplostim Using Updated Data from the RAISE Study
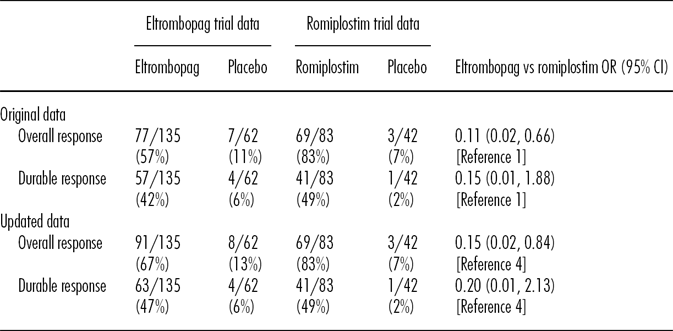
Note. Odds ratios were calculated using Bayesian methodology (1,4).
OR, odds ratio; CI, confidence interval.
Response definitions in the romiplostim trials: overall platelet response was the percentage of patients with a platelet count ≥50 × 109/L for at least 4 weeks during the trial, excluding responses within 8 weeks after rescue medications; durable platelet response was defined for romiplostim as the percentage of patients with platelet count ≥50 × 109/L on at least 6 of the last 8 weeks of treatment, with no rescue medications at any time during the trial (1). Response definitions in the eltrombopag trials: overall platelet response was a durable or transient response; transient response was platelets 50–400 × 109/L for ≥4 consecutive weeks during treatment including all data up to time of withdrawal for patients who prematurely withdrew, excluding responses during rescue treatment and up to the time platelet counts fell below 50 × 109/L after cessation of rescue treatment; durable platelet response was platelets 50–400 × 109/L for at least 6 of the last 8 weeks of treatment, excluding premature withdrawals and patients using rescue therapy at any time on treatment (3).
Several alternative methods are available for conducting an indirect treatment comparison. Our original article presented analyses using five methods, incorporating either a Bayesian or Bucher approach, and in each case the results indicated that romiplostim significantly improves overall platelet response compared with eltrombopag. We consider the Bayesian method to be a more robust approach than the Bucher method, because the Bayesian method includes all data in a single model that accounts for the heterogeneity between studies and preserves the within-trial randomization. The NICE Evidence Review Group (ERG) also considered the Bayesian analysis to be most appropriate, and used this approach in their review of the NICE eltrombopag submission (Reference Cummins, Fielding and Scott4). Including the new eltrombopag response data, and using the same Bayesian methodology as previously used by us, the ERG found that the results remained consistent with our original analysis (Table 1): the overall platelet response was significantly higher in patients receiving romiplostim than in those receiving eltrombopag (odds ratio [OR], 0.15; 95 percent confidence interval [95%CI], 0.02–0.84), assuming medium heterogeneity. Also consistent with our original analysis, the ERG found that while the point estimate favored romiplostim, there was no statistically significant difference in durable response between eltrombopag and romiplostim (OR, 0.20; 95% CI, 0.01–2.13).
Results from indirect treatment comparisons between eltrombopag and romiplostim should be interpreted with caution due to heterogeneity between the study designs, patient populations, and response definitions. Nonetheless, in the absence of head-to-head studies these analyses provide important evidence on the relative efficacy of the two currently available thrombopoietin-mimetics in patients with ITP. Using the same Bayesian approach as in our original study, an independent research group on behalf of NICE (the ERG) have used updated evidence to demonstrate that the overall platelet response remains statistically significantly greater with romiplostim than with eltrombopag.
CONFLICTS OF INTEREST
Katy Cooper and Ron Akehurst report their institute has received funding from Amgen to undertake the original research in reference 1. James Matcham and Kawitha Helme are employed by Amgen and own stock and/or shares in the company. James O'Kelly, employed by Amgen Ltd, provided medical writing assistance.



