Summary of the reasons why our paper is useful to the readership
We believe our manuscript is useful and of clinical relevance to the readers of IPG. Vascular dementia (VD) is the second commonest cause of dementia and the last paper to investigate mortality in young-onset and late-onset VD was published in 2008. In 2019, there were a few papers which compared mortality in young-onset VD and other dementias, but there has not been a paper which specifically addresses the risk factors to mortality in young- and older-onset VD. The questions “How long do I have?” and “what can I do about it?” are frequent questions following a diagnosis of dementia. Having contemporaneous information about survival duration and risk/preventive factors to mortality is important for individuals with dementia and their families, as well as for future planning and service provision.
Introduction
Vascular dementia (VD) is one of the more common types of dementia, thought to be the second most common cause after Alzheimer’s disease (Wallin et al., Reference Wallin, Milos, Sjogren, Pantoni and Erkinjuntti2003). The diagnosis of VD encompasses a range of heterogeneous entities in which vascular pathophysiological processes contribute to the clinical presentation of dementia. Consequently, there are different diagnostic criteria by which to define VD, including those proposed by the National Institute of Neurological Disorders and Stroke-Association Internationale pour la Recherche et l'Énseignment en Neurosciences (NINDS-AIREN), International Classification of Diseases, 10th Revision (ICD-10), Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM-IV), and Alzheimer’s Disease Diagnostic and Treatment Centers (ADDTC) (American-Psychiatric-Association, 1994; Chui et al., Reference Chui, Victoroff, Margolin, Jagust, Shankle and Katzman1992; Roman et al., Reference Roman1993; World-Health-Organization, 1992). When these different criteria are compared, it is evident that the diagnostic entity of VD is a complex and heterogenous, for which pathophysiology may be multifactorial, encompassing a range of different patient populations (Wetterling et al., Reference Wetterling, Rold-Dieter and Karl-Jochen1996).
The Hachinski score is one method of identifying VD (Hachinski et al., Reference Hachinski1975). The original study examined 24 patients who were assessed for clinical features of “multi-infarct dementia,” and using this score, the authors were able to divide the patients into two groups, one with “multi-infarct dementia” who scored ≥7 and one with “primary degenerative disorder” who scored ≤4. There are 13 items in the original Hachinski score, which included abrupt onset, history of hypertension and strokes, nocturnal confusion, depression, somatic complaints, and focal neurological symptoms and signs. Five items are given double weighting, with a maximum score of 18. A subsequent paper has described Hachinski scores of 7 and 5 items, respectively (Hachinski et al., Reference Hachinski, Oveisgharan, Romney and Shankle2012).
Young-onset dementia (YOD) refers to a dementia where symptom onset occurs before 65 years old (Rossor et al., Reference Rossor, Fox, Mummery, Schott and Warren2010). YOD affects over 28,000 people in Australia (Brown et al., Reference Brown, Hansnata and La2017) and can place significant burden on the individuals and families who are affected by this illness—including implications related to financial needs and emotional distress (Kang et al., Reference Kang, Farrand, Walterfang, Velakoulis, Loi and Evans2022). Individuals with YOD have complex needs for which timely diagnosis is vital to providing appropriate care (Draper et al., Reference Draper2016; Loi et al., Reference Loi2022a). Alzheimer’s disease (AD) is the most common cause of YOD, with frontotemporal dementia (FTD) and VD also being frequently diagnosed (Ferran et al., Reference Ferran, Wilson and Doran1996; Fujihara et al., Reference Fujihara, Brucki, Rocha, Carvalho and Piccolo2004; Loi et al., Reference Loi2021). For future planning and service provision, knowledge of survival duration and risk factors to mortality is important, but there has been less focus on YOD compared to late-onset dementia (LOD). In particular, there is sparse literature investigating survival in young-onset vascular dementia (YO-VD), a dementia with known risk factors that can potentially be modified.
Investigation into survival for VD has mostly involved individuals older than 65 years old (i.e., late-onset vascular dementia; LO-VD), with a median survival of approximately 3.3 years (Wolfson et al., Reference Wolfson2001). Those who have had a stroke are reported to have a shorter survival of 2.6 years (Knopman et al., Reference Knopman, Rocca, Cha, Edland and Kokmen2003). The same study reported LO-VD to have a shorter survival compared to late-onset Alzheimer’s dementia (LO-AD), related to vascular comorbidity.
In the few studies to date, people with YO-VD have longer survival compared to that found for people with LO-VD. A study comparing 48 people with YO-VD with 179 people with young-onset Alzheimer’s dementia (YO-AD) found median survival to be 6.1 years with no significant difference between the dementias (Kay et al., Reference Kay, Forster and Newens2000). The study found that a higher Hachinski score predicted mortality in YO-VD. The study used a national death registry to follow-up all the cases from 1985 to 1989 over seven years. A cohort-control study examined the survival of 4495 patients with different types of dementia at different ages from 2000 to 2014 and included 44 individuals with YO-VD and 121 individuals with LO-VD (Rhodius-Meester et al., Reference Rhodius-Meester2019). The authors also used a death registry (accessed in 2017) and found an overall median survival time from dementia diagnosis to be 6.0 years. Survival for LO-VD was 4.1 years, but median survival was not calculated for the YO-VD group as less than 50% had died.
A much longer median survival for YO-VD was reported in a prospective study of 198 patients with various subtypes of YOD, including 34 with YO-VD, 122 with YO-AD, and 42 with young-onset frontotemporal dementia (YO-FTD) (Gerritsen et al., Reference Gerritsen2019). Those with YO-VD, YO-AD, and YO-FTD had a median survival of 14.6 years, 8.6 years, and 11.3 years after diagnosis, respectively. Unlike the two previous studies, death information was determined from case notes with 21% attrition. It was unclear which YOD subtype these individuals who were lost to follow-up belonged, possibly contributing to the longer survival times. Despite this limitation, the study identified age at symptom onset to be associated with survival in YOD; with the likelihood of a shorter survival increasing by 14% with each additional year of age at symptom onset. This increased likelihood of shorter survival with age was found in all subtypes of YOD, including YO-VD.
Our recent study investigated mortality in a range of dementia subtypes of various ages and found that LO-VD had a median survival of 5.3 years compared to YO-VD, 12.3 years, from age of onset (Loi et al., Reference Loi2022c). The objectives of this study were to expand on these results to: (1) compare the clinical variables, including the Hachinski score, between inpatients admitted with YO-VD and LO-VD; (2) investigate median survival in YO-VD and LO-VD; (3) investigate predictors of association of mortality in YO-VD and LO-VD; and (4) estimate the risk of mortality in these individuals compared to the Australian population.
Methods
Study setting
This was a retrospective file review of patients who were admitted to Neuropsychiatry, The Royal Melbourne Hospital, from 1992 to 2014 inclusive, and who were diagnosed with VD using the Diagnostic Statistical Manual of Mental Disorders, 4th Edition (American-Psychiatric-Association, 1994). Neuropsychiatry is a tertiary specialist referral center which provides assessment, diagnosis, and follow-up of people with a range of neuropsychiatric conditions, including YOD (Loi et al., Reference Loi, Walterfang, Kelso, Bevilacqua, Mocellin and Velakoulis2022d). Patients are admitted for comprehensive review, comprising of neuropsychiatry, neurology, neuropsychology, neuroimaging, and biological assessments. Diagnoses are made using consensus criteria, following multidisciplinary consideration. For more information of this methodology, please refer to our recent manuscript which includes this cohort (Loi et al., Reference Loi2022c).
Data collection
MJY and MK performed the initial file review. Clinical variables collated included sex, age of onset of symptoms related to dementia, age at admission, and time course of symptoms. Vascular risk factors were identified and included history of hypertension, diabetes, stroke, hypercholesterolemia, and smoking status. In order to rate the Hachinski score, additional variables were included: presence of nocturnal confusion, preservation of personality, diagnosis of depression, presence of somatic complaints, emotional incontinence, and presence of focal neurological signs or symptoms. These clinical variables were then used to calculate the full/13-item, 7-item, and 5-item Hachinski scores (Hachinski et al., Reference Hachinski1975; Hachinski et al., Reference Hachinski, Oveisgharan, Romney and Shankle2012). Inter-rater variability between MJY and MK was addressed by discussion with an independent reviewer, SL. Reports of structural neuroimaging using magnetic resonance imaging (MRI) were reviewed and codified for data entry. “Vascular changes” were indicated as present if the MRI report stated phrases such as “lacunar infarcts,” “stroke,” “deep white matter change,” “chronic ischemic change,” “white matter disease,” “periventricular white matter disease,” or “small vessel ischemia.” Cognition was measured using the Neuropsychiatry Unit Cognitive assessment (NUCOG) which evaluates five domains of cognition, attention, visuospatial and executive function, memory, and language, with a maximum score of 20 in each domain (Walterfang et al., Reference Walterfang, Siu and Velakoulis2006). Mortality information, including age at death and cause of death, were obtained from the Australian Institute of Health and Welfare (AIHW) National Death Index, with linkage date being September 30, 2019 (Australian Government, 2019). One hundred of all names submitted were identified. Standardised mortality ratios (SMR) were used to compare mortality with published Australian Bureau of Statistics data age-sex-specific population norms (Australian-Bureau-of-Statistics, 2019).
Statistical analysis
Statistical analyses were performed using Stata (Statacorp, version 6) and Statistical Package for Social Sciences (IBM Corporation, version 26). Survival was defined as the time from symptom onset until the date of death. The Kaplan–Meier method was used to estimate median survival with the log rank test used for comparison. Cox proportional hazards ratio was used to analyze for predictors of survival. Variables which had p < 0.2 in the univariable model were included in the multivariable Cox regression model.
Ethical approval was provided by Melbourne Health (2016.037) and the AIHW human research ethics committees (EO2017/5/398).
Results
Demographics
There were 84 patients diagnosed with VD, of whom 45 (53.6%) were male and 39 (46.4%) were female. Overall, the mean age of onset for the cohort was 63.7 years (SD = 9.9, range 38, 83). Fifty-seven (67.9%) were admitted from home and half (53.6%) had obtained secondary education. The majority (96.4%) were categorized as having Oceanic/Northern Western and Southern Eastern European ethnicity (Australian-Bureau-of-Statistics, 2019). Of those who had a NUCOG (n = 36), the mean total score was 61.0 (SD = 17.1, range 32, 88), indicating moderate cognitive impairment. There were 70 (83.3%) patients who had completed MRIs during their admission and of these, 59/70 (84.2%) had “vascular changes.” The average number of vascular risk factors was 2.5 (SD = 1.5, range 0, 7). The total score for the full 13-item Hachinski was 7.6 (SD = 2.8, range 2, 14), modified 7-item Hachinski was 2.8 (SD = 1.4, range 0, 6), and modified 5-item Hachinski was 2.1 (SD = 0.8, range 1, 4).
Using symptom onset less than 65 years old to designate YO-VD, 43 patients (51.2%) had YO-VD and 37 (48.8%) had LO-VD, with 4 individuals unable to have their age of onset determined from the file notes. Table 1 shows the clinical-demographic information.
Table 1. Demographics of the sample, n = 84 patients with vascular dementia

LO-VD, late-onset vascular dementia; NUCOG, Neuropsychiatry Unit Cognitive assessment; SD, standard deviation; YO-VD, young-onset vascular dementia.
*p < 0.001.
Comparing YO-VD and LO-VD
There were differences in several of the clinical variables between those with YO-VD compared to those with LO-VD including the age at admission (YO-VD mean 60.3 years ± 5.9, vs. LO-VD mean 75 years ± 4.3; t (76) = 12.86, p < 0.001) and age at death (YO-VD mean 67.6 years ± 5.7, vs. LO-VD mean 79.8 years ± 5.9; t (58) = 8.31, p < 0.001). LO-VD had significantly higher Hachinski scores, including the full Hachinski scores (YO-VD mean 6.9 ± 2.8, vs. LO-VD mean 8.5 ± 2.7; t (76) = 2.57, p = 0.012), and the 5-item Hachinski scores (YO-VD mean 1.9 ± 0.8, vs. LO-VD mean 2.4 ± 0.8; t (77) = 2.66, p = 0.009). There were no differences in alcohol use, number or type of vascular risk factors, or in the 7-item Hachinski scores. There were no differences in vascular changes reported on MRI between LO-VD and YO-VD (χ(2) = 0.745, p = 0.388).
Mortality
Of the 84 patients, 68 (81%) had died at time of data linkage. The mean age of death was 74.1 years (SD = 8.2, range 53.5, 91.7). There were no differences in clinical variables between those who had died and those who were still alive, except for the full Hachinski score (alive mean 6.2 ± 2.5; dead mean 8.0 ± 2.8, t (82) = −2.439, p = 0.022), and modified 7-item Hachinski score (alive mean 2.0 ± 1.3; dead mean 2.9 ± 1.4, t (82) = −2.319, p = 0.029), indicating that those who had died had a higher Hachinski score. Of those who were deceased, there was a higher proportion who had LO-VD (35/37; 94.6%) compared to those who had YO-VD (29/43; 67.5%); χ(2) = 14.2, p = 0.002.
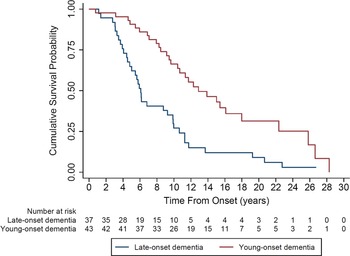
The Kaplan–Meier curves demonstrated that the median survival of the total cohort was 9.9 years (95% CI 7.9, 11.7). Those with LO-VD had significantly shorter median survival (6.1 years; 95% CI 4.8, 9.8) compared to those with YO-VD (12.8 years, 95% CI 9.6, 18.0); p = 0.03 (Figure 1).

Figure 1. Kaplan–Meier curve comparing median survival for young-onset vascular dementia to late-onset vascular dementia.
For the Cox regression, smoking status, hypertension, and number of vascular risk factors had univariable p < 0.2, and these were included in the final multivariable models. Age of onset was included, and three models were generated, using each of the Hachinski scores, the full score, and modified 7-item and 5-item (Tables 2, 3 and 4). The only significant predictor of mortality was older age (hazard ratio = 1.06, p = 0.001), suggesting that for every increased year of age of onset, there was an approximately 6% increased risk of death. For example, age of onset of dementia is 70 years old, compared to age of onset at 60 years old, there is 10-fold increase in risk of death with the older age of onset. When “YO-VD” was added as a variable rather than “age of onset,” then having YO-VD conferred half the risk of death compared to having LO-VD (hazard ratio = 0.50, p = 0.019, data not shown).
Table 2. Cox regression analysis for predictors of association for mortality in vascular dementia, using Full Hachinski score

# Reference Yes.
* Reference Yes.
Table 3. Cox regression analysis for predictors of association for mortality in vascular dementia, using modified 7-item Hachinski score

# Reference Yes.
* Reference Yes.
Table 4. Cox regression analysis for predictors of association for mortality in vascular dementia, using modified 5-item Hachinski score

# Reference Yes.
* Reference Yes.
Comparing the risk of having a LO-VD or YO-VD with population norms, these conferred a SMR of 2.7 (95% CI 1.9, 3.8) and 5.6 (95% CI 3.9, 8.0), respectively. Table 5 shows the breakdown with age categories, such that having YO-VD at 60–69 years, had 7.7× increased risk of death compared to the general population.
Table 5. Standardised mortality ratios (SMRs) for people with vascular dementia compared to population norms

CIs, confidence intervals; LO-VD, late-onset vascular dementia; SMR, standardized mortality ratio; YO-VD, young-onset vascular dementia.
Discussion
This is the most recent study to date which investigates survival and risk factors to mortality in YO-VD and LO-VD. We used a national death index and data linkage to minimize attrition and had sample sizes of YO-VD similar to previous studies. Our main findings were (1) median survival for LO-VD was 6.1 years and for YO-VD 12.8 years, (2) the only significant predictor of mortality was increasing age, and (3) having VD conferred a high risk of mortality compared to the general population; approximately 3× for LO-VD and 6x for YO-VD.
The findings of median survival duration of about 6 years for age of onset for LO-VD and 12 years for YO-VD are comparative to previous studies taking into consideration diagnostic delay (Kay et al., Reference Kay, Forster and Newens2000; Knopman et al., Reference Knopman, Rocca, Cha, Edland and Kokmen2003). In contrast, Gerritsen et al. (Reference Gerritsen2019) reported 14 years median duration for YO-VD. They attributed this longer median duration to very early identification of symptom onset.
While there were higher Hachinski scores (full and 5-item) in individuals with LO-VD, the Hachinski score itself was not a predictor for mortality. We did not find any other significant clinical variables which differed between the LO-VD and YO-VD groups. As far as we are aware, this is the first study to investigate these clinical differences. Increasing age is a risk factor for death and is an independent risk factor for cardiovascular disease (Dhingra et al., Reference Dhingra, Ramachandran and Vasan2012). Our finding that age was the only predictor of mortality emphasizes the important role that age plays in death, rather than the type or number of vascular risk factors. Increasing age is consistently found as a risk factor for mortality in dementia in many studies (Brodaty et al., Reference Brodaty, Seeher and Gibson2012; Guehne et al., Reference Guehne, Riedel-Heller and Angermeyer2005; Heath et al., Reference Heath, Mercer and Guthrie2015; Todd et al., Reference Todd, Barr, Roberts and Passmore2013). We found that for every additional year for age of onset, the risk of death increased by 6%; Gerritsen et al. (Reference Gerritsen2019) reported their increased risk as 14%.
We had anticipated that using a measure of VD such as the Hachinski score, which has been found as a risk factor for YO-VD (Kay et al., Reference Kay, Forster and Newens2000), would yield a positive result, but our results did not show this. There are limitations of the Hachinski scale (Dening and Berrios, Reference Dening and Berrios1992; Swanwick et al., Reference Swanwick, Coen, Lawlor, OʼMahony, Walsh and Coakley1996) such as the double weighting of some items compared to others, the limited guidance on the use of certain terms, such as “abrupt onset” and “depression,” which may vary depending on the nosology and definition that is used. Other items such as “relative preservation of personality” and “emotional incontinence” may be susceptible to high inter-rater variability, due to their subjective nature. The inclusion of both “focal neurological symptoms” and “focal neurological signs” as separate items, both of which are double-weighted, may over-represent the same measure when the total score is calculated using the 13-item version. Furthermore, according to the initial paper, perhaps the score should be considered a binary measure, rather than a continuous one.
We attempted to include neuroimaging vascular changes as reported by radiologists using a similar methodology to Knopman et al. (Reference Knopman, Rocca, Cha, Edland and Kokmen2003). There are more sophisticated methods of quantifying MRI markers of cerebrovascular disease, such as measurements of cortical and subcortical infarcts and using a hyperintensity segmentation tool to quantify white matter hyperintensities (Yassi et al., Reference Yassi2020). We were unable to do this due to MRIs performed prior to 2000 being only available in “hard format” and inaccessible to us and the heterogeneity of the quality of MRIs (for example, 1.5 vs 3.0 T).
Overall, we found a high risk of death compared to the general population for LO-VD (∼3×) and YO-VD (∼6×). These SMRs were similarly found in our study of mortality in overall dementia (Loi et al., Reference Loi2022c) and lower compared to what has been found for FTD, SMR = 8.1 (Loi et al., Reference Loi2022b). This highlights the high lethality of dementia in younger people and the need be able to identify these individuals as early as possible so that they can access services and support (Cations et al., Reference Cations, Loi, Draper, Swaffer, Velakoulis and Goh2021).
While the strengths of our study included a moderate sample size for LO-VD and YO-VD and no attrition for follow-up of mortality data, the retrospective nature of this study means that the clinical information obtained was limited. In addition, being a tertiary Neuropsychiatry service, the patients admitted to our inpatient unit may have different characteristics such as more psychiatric or behavioral issues, limiting the generalizability of our findings to all individuals with VaD. We were unable to collect specific details on vascular risk factors such as duration or severity, which may influence survival in VD and dementia. For example, onset of diabetes mellitus before middle age and after 15 years duration has been associated with earlier onset of dementia and decreased survival (Zilkens et al., Reference Zilkens, Davis, Spilsbury, Semmens and Bruce2013). Weight/body mass index and chronic kidney disease are also potential risk factors we were unable to analyze due to inconsistency of these reports in the clinical summaries (Heath et al., Reference Heath, Mercer and Guthrie2015). Onset of VD being temporally related to stroke appears to be a significant risk factor for mortality in LO-VD (Knopman et al., Reference Knopman, Rocca, Cha, Edland and Kokmen2003), but we were also unable explore this due to limited sample size and variable documentation in the clinical summaries.
Lastly, when investigating a construct such as VD, we acknowledge that different criteria have been used over the decades including NINDS-AIREN, ICD-10, DSM-IV, and ADDTC, of which the NINDS-AIREN may be perceived as the most “strict” diagnostic criteria (Korczyn et al., Reference Korczyn, Vakhapova and Grinsbery2012). VD is clinically heterogeneous, with the onset being acute or insidious, with improvement or decline in a stepwise or gradual deterioration. There is also lack of accepted neuropathological criteria, compared to other dementias such as AD and FTD. It is possible that we have overestimated our diagnoses of VD in our study compared to other studies as we used the DSM-IV criteria. We also did not have access to post-mortem brains in order to review pathological processes and the presence of comorbidities such as AD. Because our study spanned two decades, it is conceivable that survival in dementia, including VD, may change over time due to improved control of vascular risk factors and access to anti-dementia medications such as cholinesterase inhibitors. However, Rhodius-Meester et al. (Reference Rhodius-Meester2019) adjusted for this and did not find any differences in survival for YOD over the decades, and the systematic review by Brodaty et al. (Reference Brodaty, Seeher and Gibson2012) also found minimal evidence for this.
In conclusion, we have reported on survival in individuals with LO-VD and YO-VD, which highlights the need for early identification and provision of support for maintaining quality of life for these patients and their families. While age is an unmodifiable risk factor, potential directions for research could focus on the impact of controlling vascular risk factors on survival in VD and investigating the effect of stroke on VD and mortality.
Conflicts of interest
The authors have no conflicts of interest to declare.
Source of funding
SM Loi is funded by a National Health and Medical Council Early Career Fellowship (GNT1138968) and the Yulgilbar Alzheimer’s Research Program. The funders had no role in the data acquisition, nor the manuscript produced.
Description of authors’ roles
MJY collected and performed data entry, data analysis, completed the literature review, drafted and wrote the manuscript. SML, MK and PT collected and performed data entry. ZC performed statistical analysis. All authors reviewed drafts of the manuscript and interpretation of the data. SML formulated the research questions and conceived the study design, assisted with data collection and data entry and supervised MJY.
Acknowledgments
We would like to acknowledge the patients and families who attend the service as well as all previous members of the clinical team. We also acknowledge the Australian Institute of Health and Welfare who provided us access to the National Death Index.