Management Implications
Potentilla recta (sulphur cinquefoil) is an invasive plant of serious concern within grasslands in the intermountain region of the northwestern United States and southwestern Canada. Control of P. recta can create a disturbance within the plant community that opens up available niches for colonization. Native species can colonize these niches if they are abundant in the existing vegetation and the soil seedbank. However, if native plant abundance is low in the plant community and the seedbank, their reestablishment within these open niches is unlikely. In response, active revegetation is necessary to establish a native plant community resistant to reinvasion of P. recta or secondary invasion by other nonnative species. In our study, assessment of the soil seedbank of three grassland sites invaded by P. recta within southeastern British Columbia, Canada, revealed a depauperate seedbank, dominant in P. recta and other nonnative species. This finding indicates active revegetation needs to be considered in management plans to mitigate P. recta reinvasion or secondary invasion by other invasive plants following P. recta control. Revegetation with a functionally diverse seed mix is often recommended, because a greater portion of the open niches will be occupied, limiting available resources for invading species to use. In our greenhouse study, the growth response of P. recta was negatively related to native plant aboveground biomass, with no differences in P. recta suppression when grown within established native plant communities composed of grasses, forbs, or grasses and forbs. However, native forbs, such as Achillea millefolium (common yarrow) that produce larger amounts of aboveground biomass may be target species to include in revegetation efforts. The establishment of a productive plant community is likely important to resist P. recta reinvasion. Further study is needed to examine whether this finding is observed under field conditions.
Introduction
Grasslands are a defining characteristic of the intermountain region of the northwestern United States and southwestern Canada; however, anthropogenic practices, including fire suppression, agriculture, and overgrazing, have altered the disturbance regime of grasslands, leading to reduced ecological resiliency (Meyer et al. Reference Meyer, Callaham, Stewart, Warren, Poland, Patel-Weynand, Finch, Miniat, Hayes and Lopez2021). Declines in ecological resiliency adversely affect a grassland system’s ability to resist invasion, providing the opportunity for nonnative species, such as sulphur cinquefoil (Potentilla recta L.), to invade and spread, altering the structure and function of native grassland communities (Endress et al. Reference Endress, Naylor, Parks and Radosevich2007; Meyer et al. Reference Meyer, Callaham, Stewart, Warren, Poland, Patel-Weynand, Finch, Miniat, Hayes and Lopez2021). This is demonstrated in the southern Rocky Mountain Trench of southeastern British Columbia, Canada, where heavy livestock grazing since the mid-1800s shifted grassland communities from a system dominant in native bunchgrasses, including bluebunch wheatgrass [Pseudoroegneria spicata (Pursh) Á. Löve], Idaho fescue (Festuca idahoensis Elmer), and rough fescue (Festuca campestris Rydb.), to a system dominant in nonnative species, including P. recta (Gayton Reference Gayton2004; Wikeem and Ross Reference Wikeem and Ross2002).
Potentilla recta is a perennial forb that can live more than 10 yr, and it produces an average of 6,000 seeds per plant annually (Dwire et al. Reference Dwire, Parks, McInnis and Naylor2006). Seeds are primarily dispersed within 3 m of the parent plant, which enables P. recta to form dense, continuous stands (Dwire et al. Reference Dwire, Parks, McInnis and Naylor2006). Seeds are dormant after maturity, with dormancy broken by a combination of soil moisture, exposure to light at the soil surface, and diurnal temperature fluctuations, after which seeds remain nondormant in the soil for more than 2 yr (Baskin and Baskin Reference Baskin and Baskin1990). Once seeds are brought to the soil surface and exposed to light, they can germinate throughout the growing season if soil moisture is nonlimiting (Baskin and Baskin Reference Baskin and Baskin1990). Baskin and Baskin (Reference Baskin and Baskin1990) found germination to be relatively high, ranging from 41% to 87% in the fall, following the afterripening of seeds during the summer. In a comparison with its native congener, slender cinquefoil (Potentilla gracilis Douglas ex. Hook.), McIver and Erickson (Reference McIver and Erickson2012) found nearly twice as many P. recta seeds germinated (35.0% vs. 19.5%), and seeds consistently germinated for over 8 mo following wetting, whereas the majority of P. gracilis seed germination occurred within 2 mo following wetting. Potentilla recta also invested three times as much resources into seed production than P. gracilis, and the proportion of buds that showed evidence of seed predation was only 0.01 compared with 0.22 for P. gracilis. The lack of seed predation, the input of seed into the seedbank, and the viability of nondormant seeds in the soil for more than 2 yr, as well as the successful germination of P. recta over a long germination window, can allow P. recta to dominate both the seedbank and the aboveground plant community over time (Dwire et al. Reference Dwire, Parks, McInnis and Naylor2006). Generally, the abundance of seeds within a seedbank decreases with soil depth; however, an understanding of the seedbank at various soil depths is valuable, because it determines whether the impact of an invasive plant, such as P. recta, is primarily at the surface layer, which is composed of the most recent seed rain, or whether its impact is on the more persistent component of the seedbank, which is present at a greater soil depth (Gioria and Pyšek Reference Gioria and Pyšek2015). Seedbanks depauperate in native species and dominated by P. recta and other nonnative species can result in P. recta remaining dominant in the plant community or lead to secondary invasion from another invasive species (Mangold Reference Mangold, Monaco and Sheley2012).
Potentilla recta was identified by Ortega and Pearson (Reference Ortega and Pearson2005) as a “strong invader,” which is an invasive plant that becomes dominant within a community at the expense of native species. Endress et al. (Reference Endress, Naylor, Parks and Radosevich2007) attributed the success of Potentilla recta invasion to past land use, as P. recta dominance was found to be 37 times greater in old fields compared with less disturbed habitats, including grassland, shrubland, open forest, and forest. The study suggests P. recta dominance is primarily a result of the interaction between P. recta invasion, disturbance, and changes to land use/land cover rather than competitive superiority (Endress et al. Reference Endress, Naylor, Parks and Radosevich2007). As such, P. recta is suggested to be a “passenger” of fundamental environmental changes that are limiting native vegetation. However, research by Maron and Marler (Reference Maron and Marler2008a, Reference Maron and Marler2008b) found P. recta invasion into monocultures of native species significantly depressed native biomass, and P. recta invasion into native plant communities reduced native biomass by an average of 22.1%. The studies by Maron and Marler indicate P. recta can successfully exert competitive dominance over native grassland species.
Although P. recta has demonstrated competitive dominance, certain native species, including tall cinquefoil (Potentilla arguta Pursh), common yarrow (Achillea millefolium L.), Wilcox’s penstemon (Penstemon wilcoxii Rydb.), and F. idahoensis, exhibit resistance to P. recta invasion, with the greatest resistance exhibited by P. arguta, followed by A. millefolium (Maron and Marler Reference Maron and Marler2008a). Invasive forbs have been considered more functionally similar to native forbs than native graminoids, suggesting native forbs are key species in suppressing P. recta, as they will directly compete with P. recta for resources in response to niche overlap (Funk et al. Reference Funk, Cleland, Suding and Zavaleta2008; Scharfy et al. Reference Scharfy, Funk, Olde Venterink and Güsewell2011). Maron and Marler (Reference Maron and Marler2008b) also found diverse plant communities were more resistant to P. recta invasion than less diverse communities, which aligns with the concept of limiting similarity, in which a diversity of native species will ensure niche space is occupied that would otherwise be invaded (Funk et al. Reference Funk, Cleland, Suding and Zavaleta2008). Funk et al. (Reference Funk, Cleland, Suding and Zavaleta2008) concluded a community that is more functionally diverse would eliminate or reduce available niches for invaders to occupy.
Potentilla recta control creates a disturbance in the plant community, which opens up niches available for colonization (Mangold Reference Mangold, Monaco and Sheley2012). Passive restoration, which relies on natural succession, is sufficient to fill the open niches if native plants are able to regrow quickly, there is an abundance of native plant propagules, and the disturbance created from P. recta control is low (Mangold Reference Mangold, Monaco and Sheley2012; Schuster et al. Reference Schuster, Wragg and Reich2018). However, if the abundance of native species is low in the existing plant community and seedbank, active revegetation is required (Mangold Reference Mangold, Monaco and Sheley2012). A literature review by Schuster et al. (Reference Schuster, Wragg and Reich2018) identified 85% of the grassland-based studies considered found revegetation reduced invasive plant performance, indicating revegetation in grasslands can effectively suppress invasive plants and limit reinvasion. As mentioned earlier, a diverse plant community has been found to provide the greatest resistance to P. recta invasion (Maron and Marler Reference Maron and Marler2008b); however, Endress et al. (Reference Endress, Parks, Naylor, Radosevich and Porter2012) found seeding with native grasses following herbicide application effectively reduced reinvasion of P. recta and the invasion of other undesirable species. A direct comparison of the resistance of native plant communities composed of grasses, forbs, or a combination of the two will provide further insight into revegetation efforts required to manage P. recta invasion. Further, manipulation of resource supply, specifically nutrients, will help determine whether fertilization may aid revegetation efforts.
Fertilization has been used as a tool in invasive plant management to encourage the establishment and growth of desirable species through soil nutrient manipulation, increasing the potential for desirable species to outcompete invasive plants (Cole et al. Reference Cole, King, Oyarzun, Dietzler and McClay2007). However, the productivity and competitive response of the invader may also increase with greater resource availability (Davis et al. Reference Davis, Grime and Thompson2000). Maron and Marler (Reference Maron and Marler2008a, Reference Maron and Marler2008b) examined how increased resource supply would alter the competitive outcome of P. recta through a water supplemental treatment and found no effect of water addition on the competitive impact of P. recta. Examining P. recta response in fertilized plant communities will provide insight into whether fertilization increases P. recta productivity or whether it enhances native plant productivity to suppress P. recta, which supports the application of fertilization in revegetation efforts to manage P. recta invasion.
The purpose of this research was to gain insight into the role of native plant communities and soil seedbank in P. recta invasion within grasslands to aid grassland recovery efforts. Through two greenhouse studies, we examined (1) the above- and belowground growth response of P. recta when grown with and without fertilizer in established plant communities of varying native plant functional groups, which included native grasses, native forbs, and native grasses and forbs combined; and (2) the prevalence of P. recta and other species in the soil seedbank at varying soil depths (0 to 5 cm, 5 to 10 cm, 10 to 15 cm) of grasslands invaded by P. recta in southeastern British Columbia, Canada. We hypothesized that P. recta suppression would be greatest in the forb-only plant community and lowest in the grass-only community and that fertilizer application would increase the growth response of both P. recta and native plants. We also hypothesized that P. recta and other nonnative species would dominate the soil seedbank but that the number of emerged seedlings from the seedbank would decrease with soil depth.
Materials and Methods
Study Area
Soil and P. recta seed for the native plant community and soil seedbank studies were collected from grasslands on Tobacco Plains Indian Band (![]() ʔa·knuqʔi ‘it) reserve (49.082622°N, 115.099911°W), located within the East Kootenay Region of British Columbia, Canada, in the southern Rocky Mountain Trench. Based on a rangeland assessment (Adams et al. Reference Adam, Ehlert, Stone, Alexander, Lawrence, Willoughby, Moisey, Hincz, Burkinshaw, Richman, France, DeMaere, Kupsch, France and Broadbent2016; Keefer Ecological Services Ltd. 2020), approximately 83% of grasslands on Tobacco Plains are unhealthy and 17% are healthy with problems. Native grasses, including needle-and-thread grass [Hesperostipa comata (Trin. & Rupr.) Barkworth], Junegrass [Koeleria macrantha (Ledeb.) Schult.], and Sandberg’s bluegrass (Poa secunda J. Presl), and the nonnative grass, Canada bluegrass (Poa compressa L.), are dominant within grasslands, while P. spicata is present but sparse. Potentilla recta is a dominant nonnative forb, with common St. Johnswort (Hypericum perforatum L.), yellow salsify (Tragopogon dubius Scop.), and spring speedwell (Veronica verna L.) common. Native forbs commonly found within the grasslands include A. millefolium, pussytoes (Antennaria sp.), and timber milk-vetch (Astragalus miser Douglas ex Hook.). Shrub cover (common snowberry [Symphoricarpos albus (L.) S.F. Blake] and rose [Rosa sp.]) is sparse, and ponderosa pine (Pinus ponderosa Lawson & C. Lawson) is encroaching. A biological soil crust layer, which is an association between soil particles, cyanobacteria, algae, microfungi, lichens, and bryophytes occurring on the soil surface, is also common throughout these grasslands. A biological soil crust is important in arid grassland ecosystems because it protects the soil surface from water and wind erosion, promotes soil stability, and increases water infiltration and retention (Belnap et al. Reference Belnap, Büdel, Lange, Belnap and Lange2001; Marsh et al. Reference Marsh, Nouvet, Sanborn and Coxson2006).
ʔa·knuqʔi ‘it) reserve (49.082622°N, 115.099911°W), located within the East Kootenay Region of British Columbia, Canada, in the southern Rocky Mountain Trench. Based on a rangeland assessment (Adams et al. Reference Adam, Ehlert, Stone, Alexander, Lawrence, Willoughby, Moisey, Hincz, Burkinshaw, Richman, France, DeMaere, Kupsch, France and Broadbent2016; Keefer Ecological Services Ltd. 2020), approximately 83% of grasslands on Tobacco Plains are unhealthy and 17% are healthy with problems. Native grasses, including needle-and-thread grass [Hesperostipa comata (Trin. & Rupr.) Barkworth], Junegrass [Koeleria macrantha (Ledeb.) Schult.], and Sandberg’s bluegrass (Poa secunda J. Presl), and the nonnative grass, Canada bluegrass (Poa compressa L.), are dominant within grasslands, while P. spicata is present but sparse. Potentilla recta is a dominant nonnative forb, with common St. Johnswort (Hypericum perforatum L.), yellow salsify (Tragopogon dubius Scop.), and spring speedwell (Veronica verna L.) common. Native forbs commonly found within the grasslands include A. millefolium, pussytoes (Antennaria sp.), and timber milk-vetch (Astragalus miser Douglas ex Hook.). Shrub cover (common snowberry [Symphoricarpos albus (L.) S.F. Blake] and rose [Rosa sp.]) is sparse, and ponderosa pine (Pinus ponderosa Lawson & C. Lawson) is encroaching. A biological soil crust layer, which is an association between soil particles, cyanobacteria, algae, microfungi, lichens, and bryophytes occurring on the soil surface, is also common throughout these grasslands. A biological soil crust is important in arid grassland ecosystems because it protects the soil surface from water and wind erosion, promotes soil stability, and increases water infiltration and retention (Belnap et al. Reference Belnap, Büdel, Lange, Belnap and Lange2001; Marsh et al. Reference Marsh, Nouvet, Sanborn and Coxson2006).
Grasslands in the East Kootenay region are characterized by very dry conditions and moisture deficits during the growing season (MacKillop et al. Reference MacKillop, Ehman, Iverson and McKenzie2018). Average summer (April to September) and winter (October to March) season temperatures from 2010 to 2020 were 16 ± 5.7 C and 1.2 ± 6.8 C, respectively (Eureka Ranger Station, MT, 48.897800°N, 115.064400°W, approximately 20 km south of the study site). Average total precipitation during the summer and winter from 2010 to 2020 was 207 ± 68 mm and 183 ± 58 mm, respectively. Grasslands are composed of glaciofluvial deposits with silt loam to loamy sand textures with high coarse-fragment content (MacKillop et al. Reference MacKillop, Ehman, Iverson and McKenzie2018). Soil is classified as orthic dark brown chernozem; however, stoniness and moisture deficiency influence soil quality (British Columbia Ministry of Agriculture, British Columbia Ministry of Environment & Climate Change Strategy 2018).
Native Plant Community Greenhouse Study
The native plant community greenhouse experiment was conducted from January to April 2020 to examine the growth response of individual P. recta plants grown with and without fertilizer within a plant community composed of (1) native grasses, (2) native forbs, or (3) native grasses and forbs (Table 1). As the study was conducted in the greenhouse, results are limited in representing how P. recta growth would respond under field conditions. The experiment was arranged as a randomized factorial design with five replicates (n = 30; 2 fertilizer levels by 3 community levels by 5 replicates). The grass and forb species selected are common native species in grasslands within the region (MacKillop et al. Reference MacKillop, Ehman, Iverson and McKenzie2018), and some, specifically F. idahoensis and A. millefolium, exhibit resistance to P. recta invasion (Maron and Marler Reference Maron and Marler2008a). Historically, P. spicata was the dominant grass species within grasslands in the region and was therefore selected to represent the greatest portion of the seed mix in the grass-only and forb and grass plant communities. Achillea millefolium was selected as the dominant forb species because of its resistance to P. recta invasion and current prevalence in grasslands throughout the region (MacKillop et al. Reference MacKillop, Ehman, Iverson and McKenzie2018; Maron and Marler Reference Maron and Marler2008a).
Table 1. Proportion of grass and forb species in the native grass and/or native forb seeding treatments within a greenhouse experiment.
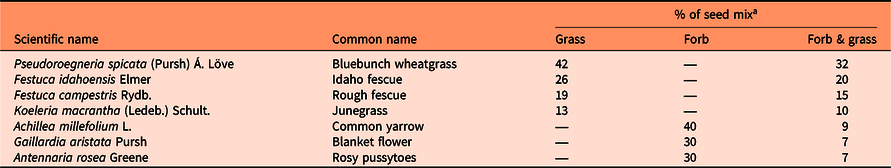
a Grass, native grasses; Forb, native forbs; Forb & grass, both native grasses and forbs. Seed was applied at a rate of 1,500 pure live seed (PLS) m −2, or 26 PLS per pot. Percentage of seed mix is based on pure live seed.
Field soil was collected in July 2019 from a field site in the northern region of Tobacco Plains reserve (49.071636°N, 115.121844°W). Five locations at this site were randomly selected, vegetation was removed, and soil was sampled to a depth of 30 cm to represent soils within the rooting zone of most plants. Collected soils were coarsely sieved to remove rocks and roots and were heated to 70 C for 40 min in an oven to thermally kill the seedbank (Dahlquist et al. Reference Dahlquist, Prather and Stapleton2007). Potential changes in soil chemistry and biota in response to soil heating were not examined. Potentilla recta seed was collected in August 2018 at the same locations where soils were collected for the seedbank study (described later). Seeds were collected from P. recta–infested areas at three random locations within three grassland sites. At each collection location, seeds from a minimum of 75 mature seed heads were shaken into plastic bags and stored at 4 C until planting. Native seed was sourced in October 2019 (Green Patch Environmental Consulting, Edmonton, AB, Canada), as local seed was unavailable, and seed was stored at 4 C until planting. In January 2020, plastic pots (15-cm top diameter and 18-cm height) were filled with a mixture of one part field soil and one part sand and saturated with water. Native grass and forb seeds, as well as fertilizer, where applicable, were dispersed on the soil surface and covered with a thin layer (0.5 cm) of soil. Seeding rate was 1,500 PLS m−2, or 26 PLS per pot. The identity of the 26 PLS per pot was the same within each given plant community treatment. An N-P-K slow release fertilizer (TerraLink Horticulture, Abbotsforb, BC, Canada), at a formulation of 14-14-14, was applied at a rate of 10 g m−2, or 0.18 g per pot. Pots were randomized on the greenhouse bench and were re-randomized once a week to account for potential differences in temperature, light, and humidity in the greenhouse. Pots were watered daily to prevent water stress. Daytime temperature of the greenhouse was 23 C and nighttime temperature was 19 C with a 17/7-h light/dark cycle.
Native plant communities (grass, forb, and grass and forb) were seeded and grown for 5 wk before transplanting four P. recta seedlings, which had been seeded and grown separately for 2 wk, into each plant community. Cover in the grass, forb, and grass and forb plant communities after 5 wk of growth averaged 42 ± 7.5%, 56 ± 9.9%, and 44 ± 8.5%, respectively. The four P. recta seedlings were grown for 2 wk within the plant communities, after which three of the four P. recta plants were removed. In each pot, the P. recta plant with the highest vitality was kept, and where individuals had equal vitality, plants were selected randomly. The remaining P. recta plant grew within each experimental treatment for an additional 6 wk and was harvested after a total of 10 wk of growth, 8 of which were in each plant community. Following the 8-wk period of P. recta growth in each plant community, the aboveground biomass of native plants was collected. The height and lateral spread of each P. recta plant was measured, and P. recta plants were subsequently removed from the pots. Potentilla recta roots were washed to remove soil, measured to determine root length (i.e., longest root), and above- and belowground biomass was separated. Native plant aboveground biomass and P. recta above- and belowground biomass were dried over 10 to 14 d at 40 C and weighed.
Soil Seedbank Study
Three sampling sites, located in the northern (49.071636°N, 115.121844°W), central (49.036689°N, 115.120292°W), and southern (49.013203°N, 115.094500°W) regions of Tobacco Plains reserve, were selected for soil seedbank analysis based on the widespread infestation of P. recta at each site. In late August 2018, three 30 by 30 cm soil pits were randomly dug at each site, and soil was collected at 5-cm increments (0 to 5 cm, 5 to 10 cm, 10 to 15 cm) for a total of 27 samples (i.e., 3 locations by 3 sites by 3 depths). Soil was air-dried and sieved through a 2-mm meshed sieve to remove roots and rocks. A 4-g subsample of soil from each sample was taken to measure ammonium (NH4 +), nitrate (NO3 −), nitrite (NO2 −), phosphate (PO4 3−), and potassium (K+) concentrations by ion chromatography using the Dionex ICS-2000 Ion Chromatography System (Sunnyvale, CA, USA). Remaining soil was stored in a −20 C freezer in darkness until further processing.
From October 2018 to February 2019, assessment of the soil seedbank was conducted using a greenhouse germination procedure. In the greenhouse, 134 g (∼30%) of soil from each of the 27 soil samples collected in the field was mixed with 250 ml of potting mix (Sunshine Mix 4, Sun Gro Horticulture, Agawam, MA, USA) and spread to a depth of 1 cm on plastic trays (34 by 21 by 12 cm). The soil mix was subsequently watered with 100 ml of water, and trays were kept at a daytime temperature of 22 C and nighttime temperature of 17 C with a 17/7-h light/dark cycle. Trays were watered twice daily to ensure optimal moisture conditions for seed germination. Over 1 mo, emerged seedlings were identified to species, counted, and removed weekly. Unidentified seedlings were grown in trays for an additional week or transplanted to a pot and grown to maturity to assist with identification. Seedlings that emerged but died before identification were counted and classified as unknown forbs or grasses. Following 1 mo, trays were air-dried for 3 d, watered with 50 ml of water, and subsequently cold stratified in a 4 C fridge for 1 mo to break seed dormancy before being returned to the greenhouse. In total, three germination–stratification cycles were conducted, as this time frame was deemed sufficient for greater than 90% of seeds to germinate (Serajchi Reference Serajchi2017).
Statistical Analysis
All statistical analyses were conducted in R v. 4.0.3 (R Core Team 2020) with a significance level of α < 0.05. A correlation analysis was conducted on P. recta above- and belowground biomass, height, lateral spread, and root length to examine relationships between these response variables. Each variable was square-root transformed to meet statistical assumptions. Analysis of covariance (ANCOVA) by the general linear model (GLM) was used to examine the effects of native plant community (grasses, forbs, grasses and forbs; fixed factor), fertilizer treatment (no fertilizer, fertilizer; fixed factor), total native plant aboveground biomass (covariate), and their interactions, on P. recta above- and belowground biomass, height, lateral spread, and root length. A linear regression was also conducted to examine the relationship between total native plant aboveground biomass and each P. recta response variable. Potentilla recta above- and belowground biomass were square-root transformed to meet model assumptions of both the ANCOVA and linear regression. A significant interaction from the ANCOVA model was assessed by the function emtrends (emmeans package).
A two-way ANOVA was used to examine the effects of native plant community, fertilizer treatment, and their interaction on total native plant aboveground biomass. A two-way ANOVA was also conducted per plant community to identify differences in aboveground biomass between native species as well as fertilizer effects. For this analysis, native species biomass was standardized by dividing biomass by the number of plants per species. Standardized biomass per plant community was square-root transformed to meet model assumptions. Tukey’s honest significant difference test was conducted on significant model results.
The species identified from the soil seedbank were divided into the following groups: P. recta, other nonnative forbs, native forbs, nonnative grasses, and native grasses. A mixed-effects general linear model (lmer function, lme4 package) was conducted per group to evaluate potential differences in number of emerged seedlings between soil depths (0 to 5 cm, 5 to 10 cm, 10 to 15 cm). Grassland site (northern, central, southern) was considered a random factor to account for site variability. A model was conducted per group due to poor model fit when all groups were included within a single model. To meet model assumptions, number of emerged seedlings was log +1 transformed and an outlier (+3 SD) from the nonnative forb group was removed. Significant model results were examined by multiple comparisons of least-squares means using the Holm-Sidak method (lsmeans function, lsmeans package, and cld function, multcomp package).
Results and Discussion
Native Plant Community Greenhouse Study
In our greenhouse study, P. recta above- and belowground biomass, height, lateral spread, and root length were correlated (R 2 > 0.80, P < 0.001). The effect of the native grass, forb, and grass and forb plant communities on P. recta growth did not differ between the communities (Table 2). This was contrary to our hypothesis that P. recta suppression would be greatest in the forb-only community and lowest in the grass-only community. In a field study conducted by Larson et al. (Reference Larson, Bright, Drobney, Larson, Palaia, Rabie, Vacek and Wells2013), functionally similar species planted to suppress Canada thistle [Cirsium arvense (L.) Scop.] within a tallgrass prairie did not negatively affect the invasive plant; rather, planted cool-season grasses and non-Asteraceae forbs reduced C. arvense cover. Larson et al. (Reference Larson, Bright, Drobney, Larson, Palaia, Rabie, Vacek and Wells2013) concluded that the establishment of early and robust native species, regardless of functional similarity, is more resistant to C. arvense invasion than establishing a functionally diverse native plant community. Under greenhouse conditions, our study suggests establishing a plant community composed of either native grasses, forbs, or a mix of grasses and forbs can resist P. recta invasion; however, high biomass production may be critical to suppress P. recta.
Table 2. Analysis of covariance (ANCOVA) of the effects of native plant community (grasses, forbs, grasses and forbs; fixed factor), fertilizer treatment (no fertilizer, fertilizer; fixed factor), total native plant aboveground biomass (covariate), and their interactions, on Potentilla recta above- and belowground biomass, height, lateral spread, and root length from a greenhouse experiment. a
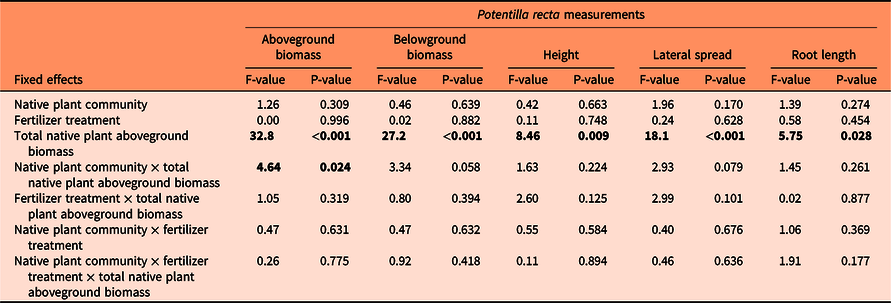
a Potentilla recta above- and belowground biomass were square-root transformed to meet model assumptions. P-values below 0.05 are bolded.
Each P. recta variable was affected by native plant aboveground biomass (Table 2). The negative relationship was most pronounced with P. recta above- and belowground biomass, with P. recta biomass declining as native plant biomass increased (Figure 1). A significant interaction between total native plant aboveground biomass and plant community was identified for P. recta aboveground biomass (Table 2), with P. recta biomass lower in the forb-only community compared with the grass-only community (Figure 1A). However, native plant biomass was higher in the forb-only community (Table 3), which likely contributed to the lower P. recta aboveground biomass measured in this community. Greater invasive plant suppression and resistance to invasion has been associated with higher biomass production in other studies (e.g., Bybee-Finley et al. Reference Bybee-Finley, Mirsky and Ryan2017; Maron and Marler Reference Maron and Marler2008b; Möhrle et al. Reference Möhrle, Reyes-Aldana, Kollmann and Teixeira2021; Sheley et al. Reference Sheley, Jacobs and Svejcar2005).
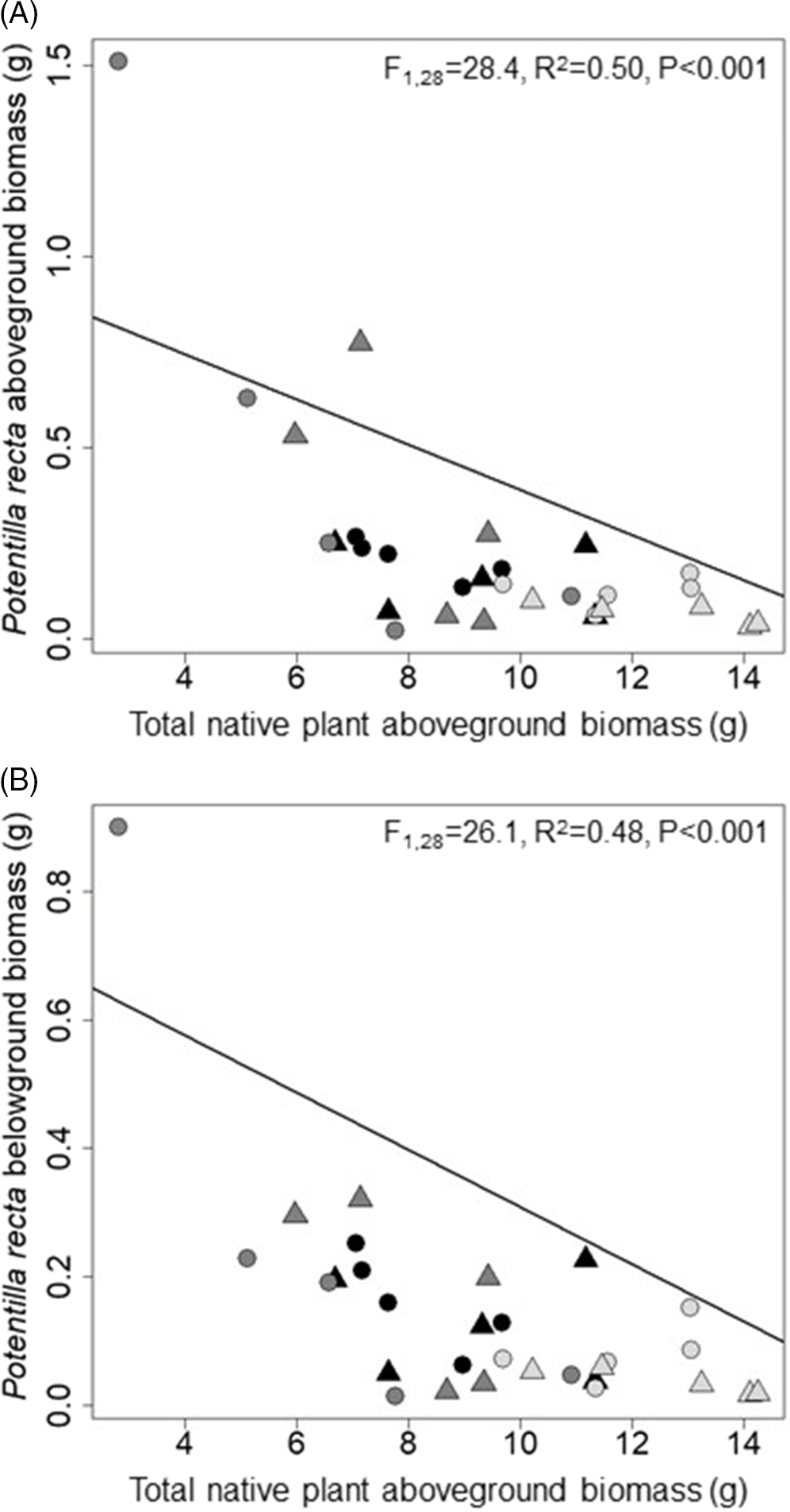
Figure 1. The relationship between total native plant aboveground biomass and Potentilla recta aboveground (A) and belowground (B) biomass in a greenhouse experiment. Triangles and circles represent data associated with fertilizer application and no fertilizer, respectively. Dark gray, light gray, and black represent data associated with the grass, forb, and grass and forb plant communities, respectively. The line represents the linear regression. The F-, R 2, and P-values obtained from the linear regression analysis are presented. Note the analysis was conducted on square-root-transformed P. recta above- and belowground biomass.
Table 3. Aboveground biomass of each plant species (mean ± SD), standardized by number of plants, and total aboveground biomass (mean ± SD) per native plant community in a greenhouse experiment. a
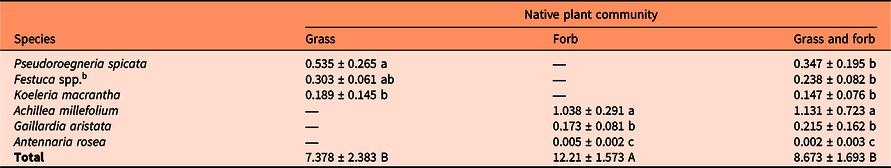
a Biomass was similar between fertilizer treatments (P > 0.05) and thus biomass presented is an average across fertilizer treatments per species and plant community. Different lowercase letters in the same column indicate significant differences in mean biomass between species (P < 0.05; ANOVA). Different uppercase letters in the total biomass row indicate significant differences in mean total biomass between plant communities (P < 0.05; ANOVA).
b Biomass of Festuca idahoensis and Festuca campestris were combined, as differentiation between the two fescues was not determined.
The inclusion of high biomass–producing plants within a seed mix may be of importance to help resist P. recta reinvasion following P. recta control within invaded grasslands. In our study, A. millefolium had the greatest biomass production, representing an average of 85% and 54% of the biomass in the forb-only and grass and forb plant communities, respectively (Table 3). Pseudoroegneria spicata followed, representing an average of 52% and 17% of biomass in the grass-only and grass and forb only plant communities, respectively. Although our study found higher biomass production might be more important in P. recta suppression and resistance to invasion than plant community composition, Maron and Marler (Reference Maron and Marler2008b) found native plant diversity played a critical role in resisting P. recta invasion in their field study. Assemblages composed of higher species richness were less invaded and less impacted than assemblages with lower species richness. Assemblages with higher diversity were also associated with higher productivity.
Fertilizer did not affect P. recta (Table 2) or native plant aboveground biomass in our study (Table 3), which was contrary to our hypothesis that fertilizer application would increase the growth response of both P. recta and native plants. Grassland soils at Tobacco Plains are generally nutrient poor. Ammonium and NO2 − concentrations were below detection (1 ppm) and NO3 − (6.9 ± 6.9 μg g−1) and PO4 3− (3.9 ± 1.1 μg g−1) concentrations ranged from deficient to low, although K+ concentrations (21 ± 26 μg g−1) ranged from moderate to sufficient (Flynn Reference Flynn2015). Fertilizer addition was expected to increase nutrient availability, positively affecting plant growth. The lack of fertilizer effect suggests nutrient availability may not have been a dominant limiting factor. In a field study conducted by Maron and Marler (Reference Maron and Marler2007), soil moisture played a significant role in predicting spotted knapweed (Centaurea stoebe L.) invasion into experimentally assembled grass and forb plant communities. Under dry conditions, C. stoebe abundance was positively associated with deep soil moisture and NO3 −, whereas under wet conditions, C. stoebe abundance was positively associated with light. Maron and Marler (Reference Maron and Marler2007) found water supplementation increased soil moisture as well as available N, which shifted competition for water and nutrients to competition for light. In our study, water was added to treatments to reduce water stress, which eliminated water as a limiting factor. In turn, light availability, rather than soil nutrients, may have become the dominant limiting factor, particularly for P. recta, as P. recta seedlings were introduced into established native plant communities. Nutrient leaching may have also occurred because of daily watering, and nutrient release may have been limited because of the application of a slow-release fertilizer. Further, soils were heated (70 C for 40 min) before the experiment to thermally kill the seedbank, which may have altered the soil chemistry and biota, influencing the observed fertilizer response.
In our study, conditions in the greenhouse were optimal for growth. In addition to nutrients, water is a limiting factor within grasslands on Tobacco Plains, which occur within a very dry climatic subregion of British Columbia (MacKillop et al. Reference MacKillop, Ehman, Iverson and McKenzie2018). Research comparing the effects of native grass, forb, and grass and forb plant communities on P. recta growth is needed under dry climatic conditions. Further, the influence of fertilizer on these native plant and P. recta communities under typical dry field conditions should be examined.
Soil Seedbank
Our soil seedbank analysis identified nine species, including five annuals and four perennials (Table 4). Seven of the species were nonnative, of which two were monocots and five were dicots, which included P. recta. Nonnative species represented approximately 60% of the average total number of emerged seedlings from the soil seedbank (Table 5). Of the average number of emerged nonnative seedlings, just over 20% were P. recta. Festuca idahoensis and pink twink [Microsteris gracilis (Hook.) Greene] were the only native species identified (Table 4), representing less than 2% of the number of emerged seedlings from the soil seedbank (Table 5). These findings support our hypothesis that nonnative species, including P. recta, would dominate the seedbank of these grasslands. The composition of the seedbank aligns with the general observation made by Rice (Reference Rice, Leck, Parker and Simpson1989) that grassland seedbanks appear to be more developed in annuals than perennials and have a larger number of forbs than grasses, with weedy species quite common. In invaded systems, depauperate seedbanks are also commonly observed (Clements and Atwood Reference Clements, Atwood and Ali2012; Gioria et al. Reference Gioria, Pyšek and Moravcova2012; Rice Reference Rice, Leck, Parker and Simpson1989). In our study, species richness in the soil seedbank was particularly low, although several emerged seedlings were unidentified (Table 5).
Table 4. Scientific name, common name, plant family, and life cycle of seedlings that emerged from the soil seedbank using a greenhouse germination procedure from soils collected on the Tobacco Plains Indian Band (![]() ʔa·knuqⱡi ‘it) reserve.
ʔa·knuqⱡi ‘it) reserve.

a , annual; P, perennial.
Table 5. Number (mean ± SD) of emerged seedlings in the soil seedbank per species from varying soil depths (0 to 5 cm, 5 to 10 cm, 10 to 15 cm) and in total (0 to 15 cm) in a greenhouse germination procedure using soils collected on the Tobacco Plains Indian Band (![]() ʔa·knuqⱡi ‘it) reserve.
a
ʔa·knuqⱡi ‘it) reserve.
a
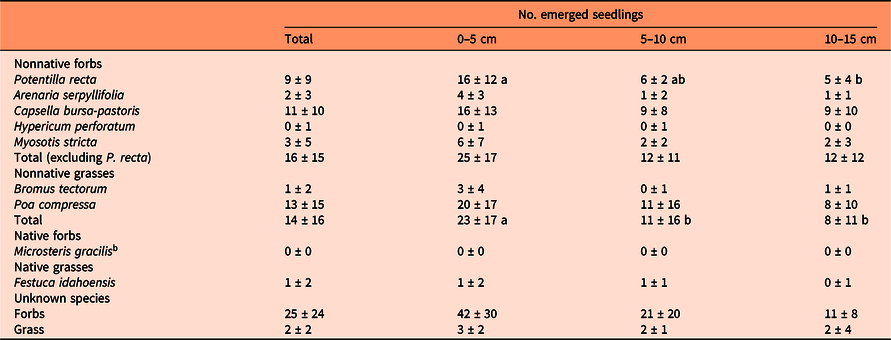
a Replicates per soil depth, n = 9. Potentilla recta and nonnative grasses had significantly higher mean number of emerged seedlings at 0- to 5-cm depth (different lowercase letters indicate significant differences, P < 0.05; ANOVA). Total number of emerged seedlings of nonnative forbs, excluding P. recta, and native grasses did not differ between soil depths. Number of emerged native forb seedlings was not compared between soil depths due to poor model fit.
b Only one emerged seedling of Microsteris gracilis was identified at 5- to 10-cm soil depth within the southern field site.
The number of P. recta emerged seedlings from the soil seedbank was lower at 10- to 15-cm soil depth compared with 0 to 5 cm (P = 0.013), with a 69% reduction in emerged seedlings (Table 5). The number of emerged nonnative grass seedlings also declined with depth (P = 0.002), with emerged seedlings reduced by an average of 59% from 0- to 5-cm to 5- to 10-cm, and 10- to 15-cm soil depths. A decline in emerged seedlings was not measured for other nonnative forbs and native grasses (other nonnative forbs: P = 0.113; native grasses: P = 0.603), and the native forb M. gracilis was only identified at the 5- to 10-cm soil depth. This did not support our hypothesis that the number of emerged seedlings from all groups would decline with soil depth. The greater number of P. recta emerged seedlings from the 0- to 5-cm soil depth was likely attributable to recent seed rain. However, the successful germination of P. recta seeds from the greater soil depths suggests viable P. recta seeds are persisting in the seedbank.
The poor representation of native species within the soil seedbank from 0 to 15 cm suggests the seedbank is degraded and/or depleted. However, we acknowledge that by selecting areas of P. recta invasion within our grassland sites, we may have biased our seedbank analysis toward invasion. Our study captured a snapshot of the soil seedbank in these invaded areas. Due to the spatial and temporal variability of seedbanks, larger sampling areas with multiple sampling periods are needed to gain a more comprehensive picture of the extent of seedbank degradation and/or depletion (Gioria and Pyšek Reference Gioria and Pyšek2015). Our study may also have underestimated viable seeds, as different species depend on varying responses to light, temperature, oxygen availability, and moisture to germinate (Baskin and Baskin Reference Baskin, Baskin, Leck, Parker and Simpson1989; Simpson et al. Reference Simpson, Leck, Parker, Leck, Parker and Simpson1989). Reviewing seed dormancy and germination requirements will help improve the seedbank analysis to better estimate species richness and density (Baskin and Baskin Reference Baskin and Baskin2014).
The Soil Seedbank and Revegetation
Efforts to control P. recta within grasslands can create an open niche within the plant community (Mangold Reference Mangold, Monaco and Sheley2012). Our soil seedbank study revealed the seedbank at our grassland sites is dominated by P. recta and other nonnative species, which indicates P. recta may reestablish within open niches created following P. recta control or secondary invasion by other nonnative species may occur (Kettenring and Adams Reference Kettenring and Adams2011; Mangold Reference Mangold, Monaco and Sheley2012). The introduction of native plant propagules through active revegetation is needed to establish a plant community that prevents P. recta reinvasion or colonization and expansion of other nonnative species from the seedbank (Kettenring and Adams Reference Kettenring and Adams2011).
Our native plant community greenhouse study suggests establishing a productive plant community may be important to resist P. recta reinvasion. Inter- and intraspecific competition were not directly examined in our study and would provide more insight into the selection of species for revegetation. In addition, findings from Maron and Marler (Reference Maron and Marler2008b) indicate a diverse plant community provides significant resistance to P. recta reinvasion. Further research that applies a similar field study design to Larson et al. (Reference Larson, Bright, Drobney, Larson, Palaia, Rabie, Vacek and Wells2013) will provide insight into whether revegetating with highly productive species is more effective at preventing P. recta reinvasion than revegetating with a species-rich seed mix (Mangold Reference Mangold, Monaco and Sheley2012).
Acknowledgments
The authors would like to give special thanks to Tobacco Plains Indian Band (![]() ʔa·knuqⱡi ‘it), and Tom Phillips in particular. Through his support, this research was able to be initiated. We also acknowledge the field and lab assistants, as well as staff of the Agriculture Greenhouses, from the University of Saskatchewan. This research was funded by the Columbia Basin Trust and the Fish and Wildlife Compensation Program. The authors declare no conflicts of interest.
ʔa·knuqⱡi ‘it), and Tom Phillips in particular. Through his support, this research was able to be initiated. We also acknowledge the field and lab assistants, as well as staff of the Agriculture Greenhouses, from the University of Saskatchewan. This research was funded by the Columbia Basin Trust and the Fish and Wildlife Compensation Program. The authors declare no conflicts of interest.









