Introduction
Despite recent advances in our conceptual understanding of alien invasive species (AIS), we still require information regarding the biological components of their life cycles and possible methods of control in order to undertake risk assessments to evaluate their impact and management options. In AIS, plasticity in traits and environmental tolerance are often thought of as important components of invasive success (Davidson et al. Reference Davidson, Jennions and Nicotra2011; Richards et al. Reference Richards, Bossdorf, Muth, Gurevitch and Pigliucci2006; Sakai et al. Reference Sakai, Allendorf, Holt, Lodge, Molofsky, With, Baughman, Cabin, Cohen, Ellstrand, McCauley, O’Neil, Parker, Thompson and Weller2001). Plastic traits allow invasive species to have a spectrum of ecological strategies to maintain fitness in a variety of environments and to benefit from a combination of different strategies (Richards et al. Reference Richards, Bossdorf, Muth, Gurevitch and Pigliucci2006). Tolerance has been shown to be an important mechanism for invasive species success (Zerebecki and Sorte Reference Zerebecki and Sorte2011) and would give them a selective advantage in disturbed areas, which are subject to high levels of light and low moisture (Sutherland Reference Sutherland2004). Several of the traits associated with AIS as agents of change are linked to resource acquisition and distribution that ultimately determine the outcome of plant interactions (Wilsey and Polley Reference Wilsey and Polley2006) and alter ecosystem functions (Vitousek Reference Vitousek1990). For example, AIS often have higher growth rates (Ramula et al. Reference Ramula, Knight, Burns and Buckley2008; Vilá and Werner Reference Vilá and Weiner2004) and a faster life cycle, increased biomass and net primary productivity, altered nitrogen fixation rates, and higher nitrogen availability than native species (Davidson et al. Reference Davidson, Jennions and Nicotra2011; Ehrenfeld Reference Ehrenfeld2003; Sutherland Reference Sutherland2004; Van Kleunen et al. Reference Van Kleunen, Weber and Fischer2009). This may lead to accumulated aboveground biomass (shoot:root ratios); for example, grass species tend to increase aboveground energy flow in the ecosystem (Wilsey and Polley Reference Wilsey and Polley2006). Even though invasive species have been shown to be highly plastic in several traits (Van Kleunen et al. Reference Van Kleunen, Weber and Fischer2009), no net fitness advantage may exist (Davidson et al. Reference Davidson, Jennions and Nicotra2011). Of the reproductive traits, clonality (Liu et al. Reference Liu, Dong, Miao, Li, Song and Wang2006) and high reproductive output (a component of propagule pressure) are characteristics that are thought to be advantageous in the invasion process (Pyšek and Richardson Reference Pyšek and Richardson2008) and are considered in risk assessments (Daehler and Carino Reference Daehler and Carino2000; Koop et al. Reference Koop, Fowler, Newton and Caton2012; Pyšek Reference Pyšek2006). For example, of the most invasive herbaceous species selected by Lowe et al. (Reference Lowe, Browne, Boudjelas and De Poorter2000) from the Global Invasive Species Database (http://www.issg.org), 72% have some form of clonal reproduction. Even though clonality is a trait that increases risk of invasion, we are still far from understanding its importance and ecological consequences (Pyšek and Richardson Reference Pyšek and Richardson2008). Clonality in AIS poses an interesting biological phenomenon, as the reduced genetic diversity makes them good subjects for exploring response to environmental tolerance. Furthermore, there are very few demographic studies that address the difference between the sexual and asexual component in invasive species (Herrera et al. Reference Herrera, Hernandez, Lanpo and Nassar2012), even though this information would be useful for management purposes, as specific stages in the life cycle can be targeted to limit population growth (Buckley 2008; Burns et al. Reference Burns, Pardini, Schutzenhofer, Chung, Siedle and Knight2013; Ramula et al. Reference Ramula, Knight, Burns and Buckley2008; Tenhumberg et al. Reference Tenhumberg, Louda, Eckberg and Takahashi2008).
Management Implications
Chandelier plant (Kalanchoe delagoensis Eckl. & Zeyh.) is a herbaceous species from Madagascar that has become invasive in many parts of the world. Introduction has been facilitated by the horticultural trade, but population growth and spread is mainly via clonal plantlets that result in monoclonal patches. However, despite the importance of plantlets, their environmental tolerance and ability to survive, which would provide information on the capacity of K. delagoensis to invade new areas, have not been evaluated. In addition, alien invasive species (AIS) can change aboveground biomass in invaded ecosystems, mainly through the differential allocation of resources. As plantlets are the main drivers of population change in K. delagoensis, we assessed their survival under greenhouse conditions with a series of herbicides (no specific treatment is currently recommended for populations in Mexico) and a control. Plantlets have a very wide tolerance to both light and water conditions, making them likely to survive in diverse habitats. Resource allocation suggests a “shooty” strategy in all conditions, meaning a strong aboveground biomass component. Of the herbicides tested, only two herbicides, glyphosate/2,4-D and 2,4-D, led to significant plantlet mortality rates in plantlets within the short time period that could potentially limit the spread of K. delagoensis. Management practices for K. delagoensis should target the plantlet stages, as they are the main source of population growth; however, in populations that also undergo sexual reproduction, management must also address reduction of seeds and seedlings.
Chandelier plant (Kalanchoe delagoensis Eckl. & Zeyh.) (Crassulaceae) is a short-lived (biennual) succulent herb native to the dry areas of Madagascar (Eggli Reference Eggli2003). Outside its native range, K. delagoensis has been listed as an AIS in South Africa (Henderson Reference Henderson2007), Australia (Batianoff and Butler Reference Batianoff and Butler2002), Cuba (González-Torres et al. Reference González-Torres, Rankin and Palmarola2012), China (Wang et al. Reference Wang, Guillot, Ren and López-Pujol2016), and Mexico (SEMARNAT 2016). Known impacts include allelopathy (Hannan-Jones and Playford Reference Hannan-Jones and Playford2002), harm to domestic animals (Capon et al. Reference Capon, MacLeod and Oelrichs1995), and changes in soil carbon (Herrera et al. Reference Herrera, Chacón, Flores, Benzo, Martínez, García and Hernández-Rosas2011). Hannan-Jones and Playford (Reference Hannan-Jones and Playford2002) found that each flower head can produce thousands of seeds with 57% germination success. Studies carried out in Mexico have shown the two species of Kalanchoe (K. delagoensis and devil’s backbone [Kalanchoe daigremontiana Raym.-Hamet & H. Perrier]) and a Houghtons hybrid (K. delagoensis×houghtonii) are each composed of a single clone and, quite possibly, the product of a single introduction event (Guerra-García et al. Reference Guerra-García, Golubov and Mandujano2015). The leaves are fleshy, with up to seven projections at the tip of each leaf that produce clonal propagules called plantlets or pseudobulbils; these drop to the ground and form new plants (Johnson Reference Johnson1934). A variety of herbicides have been considered for control of K. delagoensis: 2,4-D acid, 2,4-D amine, triclopyr+picloram, and furoxypyr (State of Queensland, Department of Agriculture and Fisheries 2016); while Benitez et al. (Reference Benitez, Loh, Tunison, Zimmer, Makaike, Mattos and Casali2012) suggest glyphosate as a possibility in Hawaii, pending control results, but mention glyphosate does affect the sister species, Kalanchoe tubiflora (Harv.) Raym.-Hamet. There are no directives in Mexico regarding what herbicide to use against species of Kalanchoe, even though it is considered an AIS (SEMARNAT 2016).
The goals of this study were: (1) to determine the survival of clonally produced plantlets to a range of water and light conditions; (2) to determine whether high levels of light, such as those often found in disturbed areas, favor clonal growth; (3) to explore different herbicides that best limit the survival of plantlets; and (4) to describe the pattern of root:shoot resource allocation in K. delagoensis individuals.
Materials and Methods
Plant Material
As part of an eradication program, whole patches of K. delagoensis individuals were collected from the grounds of the Cadereyta Regional Botanical Garden located in Cadereyta de Montes, Queretaro, Mexico (20.687061°N, 99.805255°W), in November 2011. Collected individuals represented the size structure of the population (83%: 5 to 35 cm; 13 %: 35 to 65 cm; and 4%: >65 cm). The site is located in the southern tip of the Chihuahuan Desert in central Mexico (2,044 m above sea level, 15.9 C annual mean temperature, and 488 mm annual rainfall; 60-yr data from the Mexican National Meteorological Service Station #22021).
Survival of Propagules (Experiment 1)
We tested the survival of plantlets exposed to different levels of water and light in November 2011. Pots were filled with a 50:50 soil–perlite mixture and watered daily to maintain saturation at 0%, 25%, 50%, and 100%. Pots were first watered to saturation and individually weighed. Water treatments were kept at their respective levels by weighing each pot and adding the corresponding water that was lost. In addition to ambient light levels, reduced levels were created by covering pots with mesh that extinguished light by 40% and 70% (measured with a LI-COR photosynthetically active radiation [PAR] sensor [LI-COR LI250A, Lincoln, NE]). The combinations resulted in 12 treatments (4 water levels and 3 light levels). Twenty-five pots containing three plantlets each were assigned to each treatment combination, for a total of 300 pots. As plantlets originate from lamina mother cells that have not lost their meristamic potential (Johnson Reference Johnson1934), the survival of whole leaves was tested as another possible component of clonal reproduction and to assess the ability of leaves to produce plantlets while detached from the main stem. Five pots containing three leaves were assigned to each of the 12 treatments, for a total of 60 pots. Plantlets and leaves were only placed on the soil surface with no further manipulation. Survival of propagules was recorded every 3 to 5 d for 60 d. Final survival of plantlets and leaves was analyzed using a generalized linear model with binomial errors in R v. 3.3.3. (R Development Core Team 2017) with water (4 levels) and light (3 levels) as factors. Experiments 1 and 2 (Exp 1 and Exp 2) were carried out inside a greenhouse with no supplemental lighting at the Metropolitan Autonomous University Biology Department.
Plantlet Production (Experiment 2)
The hypothesis that light affects the proliferation of plantlets was tested in a second experiment in which light was reduced by 0%, 40%, and 70% in pots that were kept at field capacity. Twenty-five pots were assigned to each treatment, with each pot containing five plantlets. The experiment was maintained for 5 mo, and the survival of leaves and the production of new propagules was recorded weekly. The propagules from this experiment were also used to determine biomass allocation (see Biomass Assignment).
Herbicide Treatment of Plantlets (Experiment 3)
We conducted a third experiment in which we used 800 plantlets of K. delagoensis to determine the most successful herbicidal treatment that could be used for control. There were 8 treatments with 10 replicates (pots), and 10 plantlets were sown in soil in each pot. To avoid confounding plantlet mortality due to transplant, plantlets that perished were replaced by new plantlets that were kept in soil until survival was constant for 1 wk. Herbicides were prepared (no surfactant added) as suggested by the manufacturers (Table 1) and applied once no mortality was observed, using a manual sprayer (approximate dose of 6.21 ml pot−1). Ten pots were assigned to one of the following eight treatments: (1) 50% glyphosate–50% atrazine (A/G), (2) commercial-grade glyphosate–2,4-D amine (G/2,4-D), (3) no herbicide added (C), (4) 50% Diuron/paraquat–50% glyphosate (D/G), (5) 100% 2,4-D (2,4-D), (6) 100% glyphosate (G), (7) 50% methylsulphuron/methyl–50% glyphosate (MG), and (8) 100% picloram–2,4 D mix (P/2,4-D). Pots were randomly rotated daily during the experiment, and plantlet mortality followed over a 4-wk period. To reduce observer bias, each pot was assessed twice by two independent observers. When counts differed, the specific pot was assessed again by both observers. Final survival of plantlets among treatments was analyzed through a generalized linear model with binomial errors, with herbicide treatment as a factor (8 levels) and survival (alive/dead) as the response variable.
Table 1 Treatment, active substance, commercial name, and preparation of the herbicides sprayed on plantlets of Kalanchoe delagoensis under controlled conditions.

a SIFATEC Alamo, Tlalnepantla, Mexico; Dow AgroSciences, Jalisco, Mexico.
Biomass Assignment (Root:Shoot Ratio)
Individual plants (n=994) collected in the field with a hand trowel were weighed (fresh biomass) to the nearest 0.001 g. The total area sampled was approximately 25 m2 spread over an area of 8 ha in a natural area within the botanical garden. Within 5-m2 patches growing as a monoculture, all individuals were sampled, such that biomass reflected the size structure of the population. Plants were then kept at 80 C for 48 h and reweighed, with biomass assigned to the belowground (root) or aboveground (shoot and leaves) portion. Plants from the plantlet production experiment (Exp 2) were also separated into above- and belowground biomass and weighed (wet and dry mass) to estimate the root:shoot ratio under controlled conditions and light intensities. The individuals subject to biomass assignment from Exp 2 had a 5-mo growing period in a greenhouse (same conditions as Exp 1) under three different light condition treatments using mesh (0%, 20%, and 70 % PAR extinction) and 100% water saturation. Linear regression identified the shoot:root relationship lnR w =lna+k lnS w , where R w is root dry biomass and S w is shoot dry biomass, a is the intercept, and k is the slope (Hunt et al. Reference Hunt, Causton, Shipley and Skew2002) in plants from the natural habitat. Regression, ANOVA, and Tukey tests compared root and shoot dry biomass (mg) and the root:shoot ratios, with light treatment as the explanatory variable. Differences between regressions were compared with analysis of covariance. All analyses were done in R v. 3.3.3 (R Development Core Team 2017).
Results and Discussion
Kalanchoe delagoensis has both clonal and sexual reproduction. However, the genetic study by Guerra-García et al. (Reference Guerra-García, Golubov and Mandujano2015) in Mexican populations of K. delagoensis indicated that the invasion of this species was the result of a single introduction event with a subsequent invasion driven by clonal spread. A study on the sister species Kalanchoe daigremontiana Raym.-Hamet & Perrier and [Kalanchoe pinnata (Lam.) Pers.], and specifically on K. delagoensis (J Golubov et al., unpublished data), highlighted the importance of vegetative propagation as the driver of population dynamics. Suggested management scenarios in all the studied species in which establishment depended exclusively on plantlet recruitment target reducing the survival and growth of clonal propagation (González de León et al. Reference González de León, Herrera and Guevara2016; Herrera et al. Reference Herrera, Hernandez, Lanpo and Nassar2012). Examples of successful invasion by clonal spread may be more common than once thought (Hollingsworth and Bailey Reference Hollingsworth and Bailey2000; Lambertini et al. Reference Lambertini, Riis, Olesen, Clayton, Sorrell and Brix2010; Weiguo et al. Reference Weiguo, Bingrui and Jianbo2006). Furthermore, the disadvantages associated with the lack of genetic variability (Prentis et al. Reference Prentis, Wilson, Dormontt, Richardson and Lowe2008) are probably offset by high environmental tolerance (Sakai et al. Reference Sakai, Allendorf, Holt, Lodge, Molofsky, With, Baughman, Cabin, Cohen, Ellstrand, McCauley, O’Neil, Parker, Thompson and Weller2001; but see Lee Reference Lee2002).
The results suggest that both water (χ2=309.01, df=3, P<0.01) and light (χ2=74.56, df=2, P<0.01) were important for plantlet survival, but the interaction was not significant (χ2=8.06, df=6, P=0.23; Figure 1). Treatments with higher water saturation and light extinction had the highest plantlet survival (Figure 1). Clonality also allows K. delagoensis to persist in the absence of seeds, even in harsh conditions, as plantlet survival was above 12% after 45 d with no water and full sunlight. Water seems to be one of the major factors that determine plantlet survival (Figure 1), and light plays an increasing but not linear role when adding water; the difference between 40% and 70% light extinction was a near doubling of survival when watering (Figure 1) was 0% and 25%. Under benign conditions, namely, reduced exposure to light and low water availability such as those found under the canopy of larger plants, the probability of survival was close to 1. This shows that clonal reproduction, an often ignored component of propagule pressure, could be contributing to invasion success, and in populations with sexual reproduction, invasion would be expected to be higher. Plantlets were able to tolerate a wide range of conditions (water and light availability) and are therefore able to maintain populations and potentially maximize fitness in terms of population growth as environmental conditions improve (master-of-some strategy sensu Richards et al. Reference Richards, Bossdorf, Muth, Gurevitch and Pigliucci2006). Clonal propagation is entirely through plantlets; leaves possessed no means of developing into new plants, as none survived after 9 wk and no plantlets were produced. Even though plantlets arise from leaf margins, their production is limited to standing individuals. The high survival of plantlets can be partially explained, as they possess roots and store starch, and the water provided by succulence increases the chances of survival (Johnson Reference Johnson1934) even before they detach from the leaf margin of the parent plant. The common trait of clonality found in many invasive species (Liu et al. Reference Liu, Dong, Miao, Li, Song and Wang2006) is expressed in K. delagoensis, as plantlets have high survival rates. Coupled with the genetic results of Guerra-García et al. (Reference Guerra-García, Golubov and Mandujano2015), the high environmental tolerance of plantlets seen in the present study indicates clonal propagation is the main and very likely the only driver that maintains populations of K. delagoensis.
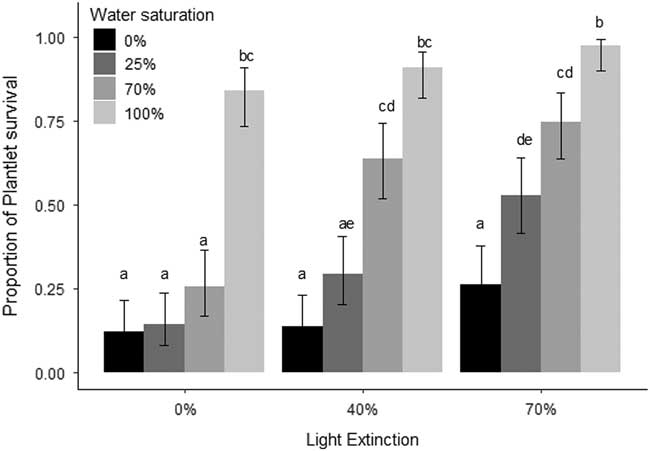
Figure 1 Proportion of surviving plantlets (±95% CI) after 60 d under a water (0%, 25%, 50%, and 100% water saturation) and light (uncovered and 40% and 70% photosynthetically active radiation extinction) treatment combination. Bars indicate 95% CI. Different letters indicate statistically significant differences between treatments.
Plantlets are generated in the leaf margins of individuals but were only produced when exposed to high light intensities (100% light: n=712 plantlets; 80% light: n=4 plantlets). Once an individual was established, there was an early onset of plantlet production as of week 5. This allocation to plantlets could potentially increase the speed at which colonization of a habitat can occur and contribute to reducing generation time.
The biomass of individuals collected in the field was highly variable (Table 2). On average, plants in natural conditions lost 83% of their biomass after drying (Table 2), and even though energetic allocation was highly correlated [r 2=0.86, F(1, 992)=3,449, P<0.0001] between shoots and roots, most resources were allocated to aboveground tissue or a “shooty” growth (k<1; Hunt Reference Hunt1990) in all cases as regression slopes did not differ significantly [F(3, 341)=69.4, P<0.01]. Mean root dry biomass (g) of plantlets from the experiment (0%, 20%, and 70% PAR extinction treatments) differed significantly between light levels and increased with light availability (Figure 2A). Mean shoot biomass, however, was significantly higher with 20% light extinction and was lowest with 70% extinction (Figure 2B). Partial shade favored growth and metabolic activity in another crassulacean acid metabolism (CAM) species, Maxocotl (Bromelia humilis Jacq.) (Medina et al. Reference Medina, Olivarez and Diaz1986), suggesting a benefit associated with biotic or abiotic objects that protect CAM plants from direct solar radiation. This is consistent with previous findings, in which mean values of root:shoot ratios were not different between the 20% and 70% light extinction treatments (Figure 2C). These results suggest that when plants are exposed to light (0% and 20% light extinction), they tend to store more water aboveground, a common trait in succulent plants and other xerophytes with shallow root systems (Barbour Reference Barbour1973; Smith et al. Reference Smith, Monson and Anderson1997).
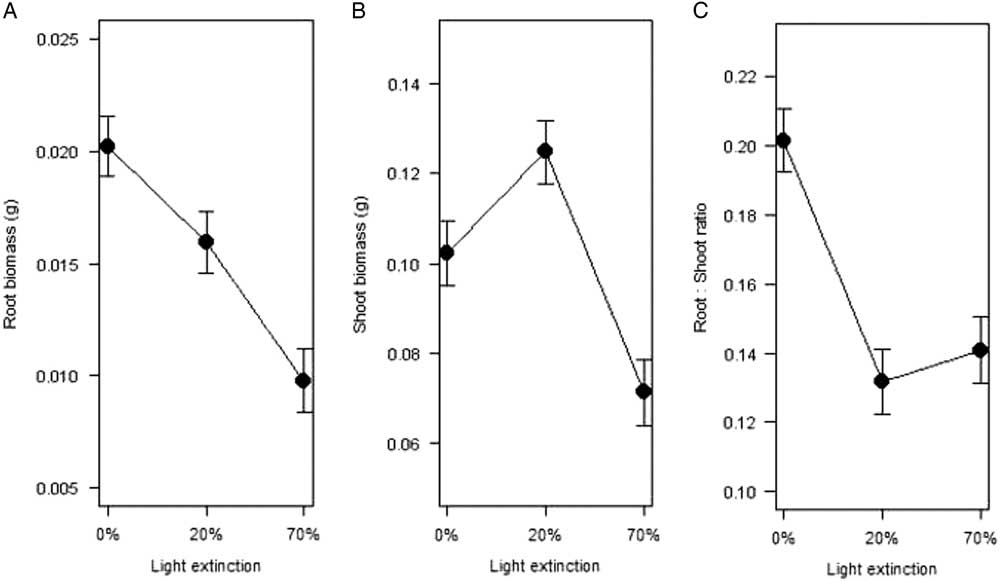
Figure 2 Mean dry biomass (g) in (A) roots and (B) shoots, and (C) root:shoot ratio for Kalanchoe delagoensis individuals kept for 5 mo under water saturation and three light conditions (0%, 20%, and 70% reduction in photosynthetically active radiation).
Table 2 Allometric root:shoot relationships (y=lna+k lnx, where y=dry root biomass and x=dry shoot biomass, k=root:shoot ratio slope, and R 2 is the correlation coefficient), and proportion of dry biomass (95% CI) from individuals of Kalanchoe delagoensis collected under field conditions and 5-mo-old plantlets subject to three light treatments.

The root:shoot ratio results support the fact that plants under light extinction assigned less biomass to underground structures, probably because they need less root biomass to balance water intake (Smith et al. Reference Smith, Monson and Anderson1997). For plantlets after 5 mo, values of the root:shoot ratio k also correspond to “shooty” growth, but exposure to high light intensities increases “rooty” growth (Table 2). According to Schulze et al. (Reference Schulze, Schilling and Nagarajah1983), the optimal ratio occurs when the plant accumulates the maximum biomass without experiencing water stress, which implies a low root:shoot ratio. With a small decrease in light (20%), individuals may be reaching the theoretical optimum proposed by Schulze et al. (Reference Schulze, Schilling and Nagarajah1983), as the ratio does not change significantly with higher light extinction (70%, P=0.368; Figure 2C). This suggests that plants under a little shade had enough PAR for photosynthesis even with a low transpiration rate, which would be further enhanced by being a CAM plant.
Root:shoot ratios from plants collected in the field were higher than those in plants from the greenhouse. This was expected, as experimental plants were water saturated, while plants under natural conditions must allocate more resources to belowground structures to capture water. Water content diminishes consistently with age and exposure to light (Table 2). In addition, most of the field-collected individuals were older (collected plants represented the entire biennual life cycle, including reproductive individuals) than those from the light experiment (only 5 mo old), and according to Shipley and Meziane (Reference Shipley and Meziane2002), root:shoot ratios may increase with age, because older roots are less efficient at absorbing water and nutrients.
Within the context of introduced species, there is no strong consensus on whether exotic species differ from natives in their above- and belowground growth rates (Wilsey and Polley Reference Wilsey and Polley2006). Pattison et al. (Reference Pattison, Goldstein and Ares1998) found no significant differences in terms of root:shoot ratio between native and invasive species in Hawaii, and Daehler (Reference Daehler2003) found no consistent differences between natives and introduced plants. Vilá and Wiener (Reference Vilá and Weiner2004) found greater aboveground rates in natives than introduced species, whereas Wilsey and Polley (Reference Wilsey and Polley2006) found lower aboveground productivity and greater biomass in roots of introduced and native grass species, probably because these introduced species were selected by humans for forage. For K. delagoensis, plants have a “shooty” growth, with less investment to root biomass as limiting conditions improve.
Clonal reproduction through plantlets is a potential target for successful control of K. delagoensis (J Golubov et al., unpublished data) as with the sister species (K. daigremontiana [Herrera et al. Reference Herrera, Hernandez, Lanpo and Nassar2012] and K. pinnata [González de León et al. Reference González de León, Herrera and Guevara2016]) they are the stage in the life cycle that can substantially decrease the intrinsic population growth rate. Survival of plantlets was nil after 2 wk using G/2,4-D amine, and 2,4-D treatment resulted in very low survival (1%), followed by five herbicide treatments (P/2,4-D, M/G, D/G, A/G, and G) that resulted in greater than 28% plantlet survival (Figure 3). The results suggest that herbicide control of K. delagoensis plantlets would be effective in the short term (<2 wk) with a G/2,4-D treatment or in the long term with a 2,4-D treatment. The glyphosate treatment suggested by Benitez et al. (Reference Benitez, Loh, Tunison, Zimmer, Makaike, Mattos and Casali2012) still yielded a 7% survival rate at the end of the experiment; the significant mortality was due to the 2,4-D amine. This suggests that mortality produced by the G/2,4-D treatment for the control of K. delagoensis is critical, because plantlets can be produced within 5 wk after being dropped from the parent plant. This means that any herbicide treatment that allows survival past 5 wk would not be as effective for the control of K. delagoensis populations but could have an impact due to a reduction in plantlet production.

Figure 3 Survival of plantlets (±95% CI) after herbicidal treatment for 2 and 4 wk. Treatments and concentrations for herbicides are described in Table 1.
The combination of a selective herbicide with glyphosate, which is a nonselective herbicide, achieves greater efficacy than the application of glyphosate alone (Norris et al. Reference Norris, Shaw and Snipes2001). Surprisingly, the P/2,4-D combination seems to be not as effective, as survival was still above 7% after 4 wk. The concentration of 2,4-D in this product (360 ai L−1) is close to that found in the product containing only 2,4-D (400 ai L−1). Even though the maximum mortality of K. delagoensis was first achieved with the G/2,4-D amine treatment, the nonselective nature of the glyphosate implies a risk of negative effects on native plants when used in the field (Laufenberg et al. Reference Laufenberg, Sheley, Jacobs and Borkowski2005). The second most effective treatment was 2,4-D, which required almost seven times less product than the combination of G/2,4-D amine (Table 1), making it a more cost-effective option. This is probably due to differing concentrations of the compound, a 160 ai L−1 concentration in G/2,4-D amine versus a 400 ai L−1 concentration for 2,4-D alone.
Plantlets of Kalanchoe have been identified as key components of the life cycle that contribute to population growth (Herrera et al. Reference Herrera, Hernandez, Lanpo and Nassar2012; J Golubov et al., unpublished data; González de León et al. Reference González de León, Herrera and Guevara2016). The environmental tolerance to light and water availability found in K. delagoensis plantlets allows the species to inhabit a wide variety of habitats. It is also very likely that the tolerance shown by K. delagoensis is shared with its sister species, and therefore the potential spread of Kalanchoe might be much larger than once thought. The high mortality of plantlets recorded using G/2,4-D amine and 2,4-D provides a viable means of chemical control that can be cost-effective and target the susceptible stages of the plant’s life cycle.
Acknowledgments
Financial support was provided by project CONABIO GN024 and GEF 089333 to JG and MCM. The authors thank Ing. Emiliano Sánchez, director of the Cadereyta Regional Botanical Garden, and staff for logistic support, access to the botanical garden, and permission to collect specimens. We also thank H. Altamirano and M. Rojas for help during field and lab work. A CONACyT sabbatical scholarship at NMSU provided support for JG and a PASPA-DGAPA-UNAM sabbatical provided support for MCM at NMSU. Two anonymous reviewers helped to improve the article. No conflicts of interest have been declared.







