Introduction
Invasive annual grasses have been shown to have devastating consequences on native biodiversity, environmental quality, and ecosystem services (Bartz and Kowarik Reference Bartz and Kowarik2019; Jones and McDermott Reference Jones and McDermott2018; Kumar Rai and Singh Reference Kumar Rai and Singh2020; Pejchar and Mooney Reference Pejchar and Mooney2009). These invasive annual grasses effectively displace native vegetation (Pyšek et al. Reference Pyšek, Jarošík, Hulme, Pergl, Hejda, Schaffner and Vilà2012), thus altering decomposition cycles and soil food webs (Lenz et al. Reference Lenz, Moyle-Croft and Facelli2003), disrupting ecosystem networks such as the plant–pollinator reproductive mutualisms (Schweiger et al. Reference Schweiger, Biesmeijer, Bommarco, Hickler, Hulme, Klotz, Kühn, Moora, Nielsen, Ohlemüller, Petanidou, Potts, Pyšek, Stout and Sykes2010; Traveset and Richardson Reference Traveset and Richardson2006), altering historic fire regimes (D’Antonio and Vitousek Reference D’Antonio and Vitousek1992), and displacing ecosystem diversity and stability (Musil et al. Reference Musil, Milton and Davis2005). Although controlling invasive species has received global and regional attention, the success of control measures and the positive impacts on the ecosystems following control may not always be uniform or generalizable (Adams et al. Reference Adams, Jennings and Warnock2020; Skurski et al. Reference Skurski, Maxwell and Rew2013) and may vary across different levels of ecological complexity (Vilà et al. Reference Vilà, Espinar, Hejda, Hulme, Jarošík, Maron, Pergl, Schaffner, Sun and Pyšek2011). It has been suggested that effective control measures for invasive species, should also emphasize ecological processes that prevent reinvasion, possibly combining control with simultaneous restoration to retain broader ecosystem functions (D’Antonio et al. Reference D’Antonio, Jackson, Horvitz and Hedberg2004; Flory and Clay Reference Flory and Clay2009; Monaco et al. Reference Monaco, Mangold, Mealor, Mealor and Brown2017).
Invasive species contribute to biodiversity losses by compounding effects of habitat destruction, agricultural intensification, and climate change, as recently discussed by Wagner (Reference Wagner2020) in a report on global decline in insect biodiversity. Although the extent of impact may vary across different ecosystems, declining populations of insects, specifically pollinators, could compromise reproductive success of native flora (Gilgert and Vaughan Reference Gilgert and Vaughan2011) and affect ecosystem functioning (Blüthgen and Klein Reference Blüthgen and Klein2011). While the impact of invasive species on native vegetation is relatively well described, and studies demonstrate targeted ecosystem trade-offs resulting from controlling invasive species (Adams et al. Reference Adams, Jennings and Warnock2020; Pyšek et al. Reference Pyšek, Jarošík, Hulme, Pergl, Hejda, Schaffner and Vilà2012; Skurski et al. Reference Skurski, Maxwell and Rew2013), few studies explore the relation between control of invasive plants and the subsequent impact on pollinator-friendly forbs. In the rangelands of Colorado, this relationship is especially critical, as the well-documented bee diversity of this region (Goldstein and Scott Reference Goldstein and Scott2015) is important for the reproductive success of the native forbs. While studies indicate that the bee populations in these rangelands may not be currently experiencing concerning declines (Kearns and Oliveras Reference Kearns and Oliveras2009b) and have been conserved over several decades (Kearns and Oliveras Reference Kearns and Oliveras2009a), the spread of invasive annual grasses could compromise the habitat quality of these rangelands, negatively impacting bee populations in the long run.
It has also been suggested that winter annual invasives such as cheatgrass or downy brome (Bromus tectorum L.) affect regional ecosystem functions (Boyte et al. Reference Boyte, Wylie and Major2016; Knapp Reference Knapp1996). A systematic review spanning 64 yr (Monaco et al. Reference Monaco, Mangold, Mealor, Mealor and Brown2017) suggests that of the different methods of control currently available, only one method, herbicide application, decreased B. tectorum and increased perennial grass abundance over the long term, lending support to herbicide-based control methods. Recent research reports from this region describe the efficient control of B. tectorum following winter application of a preemergent herbicide, indaziflam, a chemical whose cellulose biosynthesis-inhibiting action inhibits root development following seed germination, a mechanism different from previously used herbicides (Clark et al. Reference Clark, Sebastian, Nissen and Sebastian2019; Sebastian et al. Reference Sebastian, Sebastian, Nissen and Beck2016, 2017a). These studies also report that the residual effects of indaziflam application may last up to nearly three years, allowing for further reduction of B. tectorum seedbank in the soil (Sebastian et al. Reference Sebastian, Nissen, Sebastian and Beck2017b), improving the potential for native forbs to reestablish after continued germination suppression of the invasive grass seeds. Taken together, the inhibited seed germination and longer residual effect suggest that controlling B. tectorum during the winter months could improve reestablishment of the spring-emerging native flora in these rangelands (Sebastian et al. Reference Sebastian, Sebastian, Nissen and Beck2016). With this in mind, we test the hypothesis that herbicide-mediated control of B. tectorum has a positive impact on the native flora in the rangelands of Colorado by identifying the diversity and abundance of pollinator-friendly flora in the herbicide-treated and control plots. We report the findings as a case study.
Materials and Methods
Study Locations and Treatments
Three geographic locations within Boulder County, CO, shown in Figure 1 were identified for the study such that each location had paired herbicide-treated and untreated plots. Plot sizes depended on the terrain, but all plots had at least one side measuring 100 m in length. During the winter months, between December 2016 through February 2017, the area where the treated plots were demarcated received application of the preemergent herbicide indaziflam (Esplanade™, Bayer Crop Science, St. Louis, MO 63167, USA) at the rate of 102 g ai ha−1. The exact dates of application varied based on accessibility over the terrain and weather conditions. The paired treated and untreated plots were in similar habitat types with vegetation cover dominated by B. tectorum and field brome (Bromus arvensis L.; syn.: Bromus japonicus Thunb.) and 0% to 10% canopy cover of scattered co-occurring species (for a list of co-occuring species, see Sebastian et al. Reference Sebastian, Fleming, Patterson, Sebastian and Nissen2017a). The coordinates of the three locations, Rabbit Mountain Open Space West (RM 1, herbicide applied to treated plots in January 2017), Rabbit Mountain Open Space East (RM 2, herbicide applied to treated plots in February 2017), and Colp (herbicide applied to treated plots in December 2016) are shown in Figure 1. Every effort was made to ensure that the treated and control plots were in the same vicinity, but in the case of Colp, this was not feasible due to the lack of suitable locations of the required size close to the treated location. Therefore, as shown in Figure 1, the treated and control plots at Colp are farther apart than at the other two study locations, and the control plot at the Colp measured 80 by 100 m. Based on our observations during the 2018 study season, we are confident that this did not significantly affect the results being presented here.
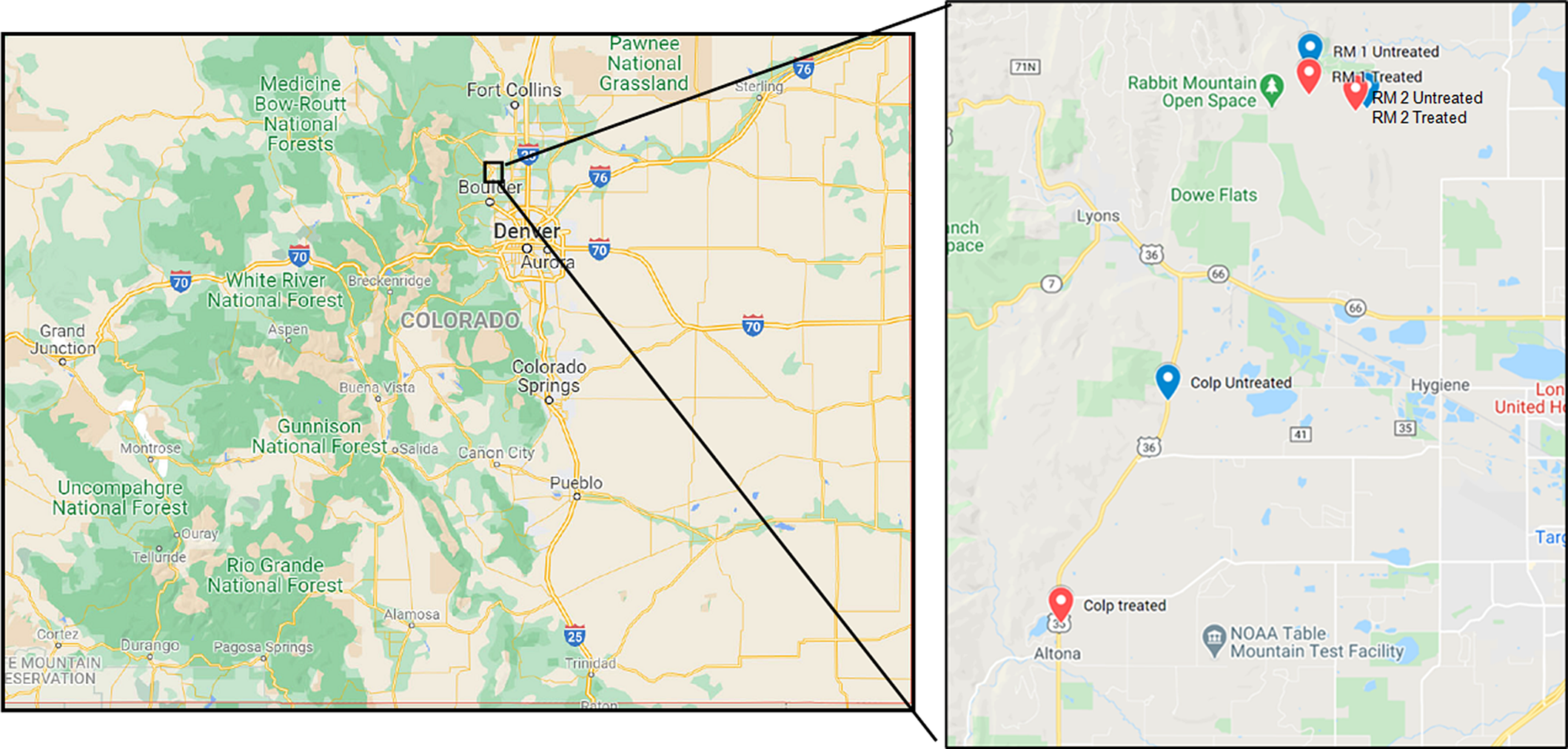
Figure 1. Study locations in Boulder County Parks and Open Space area in Colorado. State map of Colorado on the left and the inset study area on the right. The locations and their coordinates: RM 1, Rabbit Mountain 1 (Untreated: 40.2547°N, 105.2139°W; Treated: 40.2495°N, 105.2143°W); RM 2, Rabbit Mountain 2 (Untreated: 40.2468°N, 105.1984°W; Treated: 40.2463°N, 105.2015°W); Colp (Untreated: 40.1861°N, 105.2526°W; Treated: 40.1396°N, 105.2819°W).
Transect Sampling
Eight permanent 100-m belt transects were established at each survey plot, spaced evenly across the vertical and horizontal axes of the plots. A meter tape was stretched between the ends of the transects to demarcate the transect line. A 1-m2 frame was placed 1 m away from the tape at 10-m intervals, on alternating sides of the transect line. All flowering plants within the frame were identified and the number of plants of each species were counted before moving to the next frame-stop that was 10 m away. Plants that were not flowering during the sampling weeks were not recorded. When frames landed in areas with no flowering individuals, researchers moved to the next 10-m stop. For data analysis, the number of plant species in bloom was pooled across all quadrats for each transect. Sampling was conducted for a period of 8 wk (9 wk in Colp) beginning in May through September. Through the season, there were a total of 48 belt transects completed across all locations and plots. Each of the three study locations had a total of 16 belt transects, with 8 each in the treated and control plots.
Random Walk Sampling
Flowering plants that did not fall within the sampling frames of the belt transects could not be recorded during the entire study period. We conducted focal-flowering plant sampling in the entire plot using random walk sampling to obtain a census or inventory of all flowering plants that were not counted in the transect sampling. We walked the plot in an organized fashion beginning at one end spanning the entire plot, specifically targeting all flowering plants that did not fall into the frames, recording all blooming species in the plot on any given sampling day. The data collected by random walk sampling were used to create an inventory of all flowering plants recorded in our study.
Data Analyses
Standard ecological indices for plant species diversity, richness, and abundance were calculated for the treated and untreated plots for each sample event in each geographic location. Species richness is simply the total number of unique species during each sampling event. Shannon diversity index (
![]() $$H' = - \mathop \sum \nolimits_{i = 1}^R {p_i}ln{p_i}$$
) and Simpson’s diversity index (
$$H' = - \mathop \sum \nolimits_{i = 1}^R {p_i}ln{p_i}$$
) and Simpson’s diversity index (
![]() $$D = 1/\mathop \sum \nolimits_{i = 1}^R {p_i}^2$$
) were calculated as the diversity measures for pollinator-friendly flora in the three geographic locations (Magurran Reference Magurran2013; Ortiz-Burgos Reference Ortiz-Burgos2016; Pielou Reference Pielou1966; Simpson Reference Simpson1949; Whittaker Reference Whittaker1972). The Shannon diversity index combines evenness and richness into a single measure and assumes that all species are represented in a sample, while Simpson’s diversity index gives more weight to common species and assumes that the few rare ones with only a few representatives will not affect the diversity values.
$$D = 1/\mathop \sum \nolimits_{i = 1}^R {p_i}^2$$
) were calculated as the diversity measures for pollinator-friendly flora in the three geographic locations (Magurran Reference Magurran2013; Ortiz-Burgos Reference Ortiz-Burgos2016; Pielou Reference Pielou1966; Simpson Reference Simpson1949; Whittaker Reference Whittaker1972). The Shannon diversity index combines evenness and richness into a single measure and assumes that all species are represented in a sample, while Simpson’s diversity index gives more weight to common species and assumes that the few rare ones with only a few representatives will not affect the diversity values.
Data from the belt transect sampling were analyzed using a general linear model for multiple dependent variables. Treatments and geographic locations were fixed effects; sampling week was a covariate; and species richness, Shannon diversity index, and Simpson’s diversity index values were dependent variables. Treatment by location interaction was also determined to analyze any location-specific response. As needed, logarithmic transformations were performed for nonnormal species richness data before analysis.
Results and Discussion
Here we present a case study showing the richness and diversity of pollinator-friendly flora in three locations where a preemergent herbicide, indaziflam, was applied to control the invasive annual grass B. tectorum. There was a significant treatment effect on the different diversity measures. Herbicide-treated plots had higher richness and alpha-diversity measures across all three locations (Table 1; Figure 2; species richness: F(1, 41) = 23.25, P = < 0.0001; Shannon diversity index: F(1, 41) = 20.29, P = 0.001; Simpson’s diversity index: F(1, 41) = 15.87, P = 0.001), suggesting that the control of B. tectorum could result in reduced competition allowing for the reestablishment of native flowering plants. There was no significant effect of location on these measures (species richness: F(2, 41) = 0.61; Shannon diversity index: F(2, 41) = 0.08; Simpson’s diversity index: F(2, 41) = 1.09) and no significant interaction effect between treatment and location (species richness: F(2, 41) = 3.58; Shannon diversity index: F(2, 41) = 2.96; Simpson’s diversity index: F(2, 41) = 1.81).
Table 1. Multivariate general linear model showing the effect of treatment on diversity measures calculated from belt transect data.
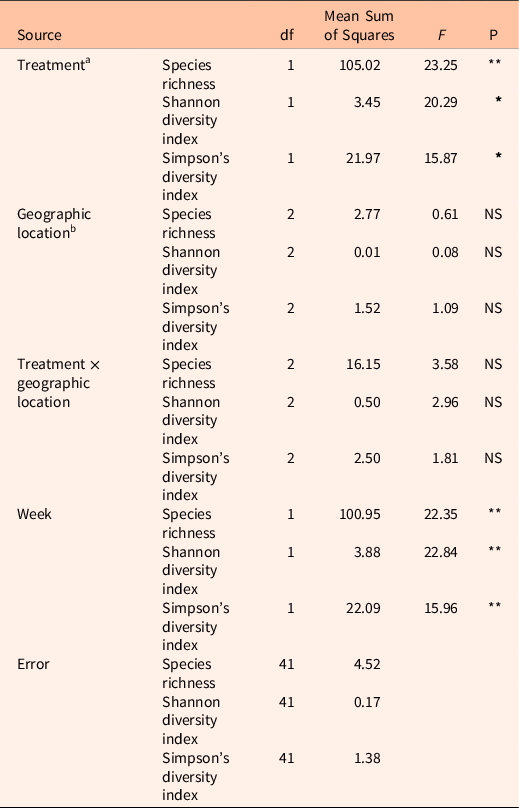
a Treatments: herbicide-treated and untreated control.
b Geographic location: Colp, Rabbit Mountain 1, and Rabbit Mountain 2.
cStatistical significance at: **P < 0.0001; * P = 0.001; NS, nonsignificant.
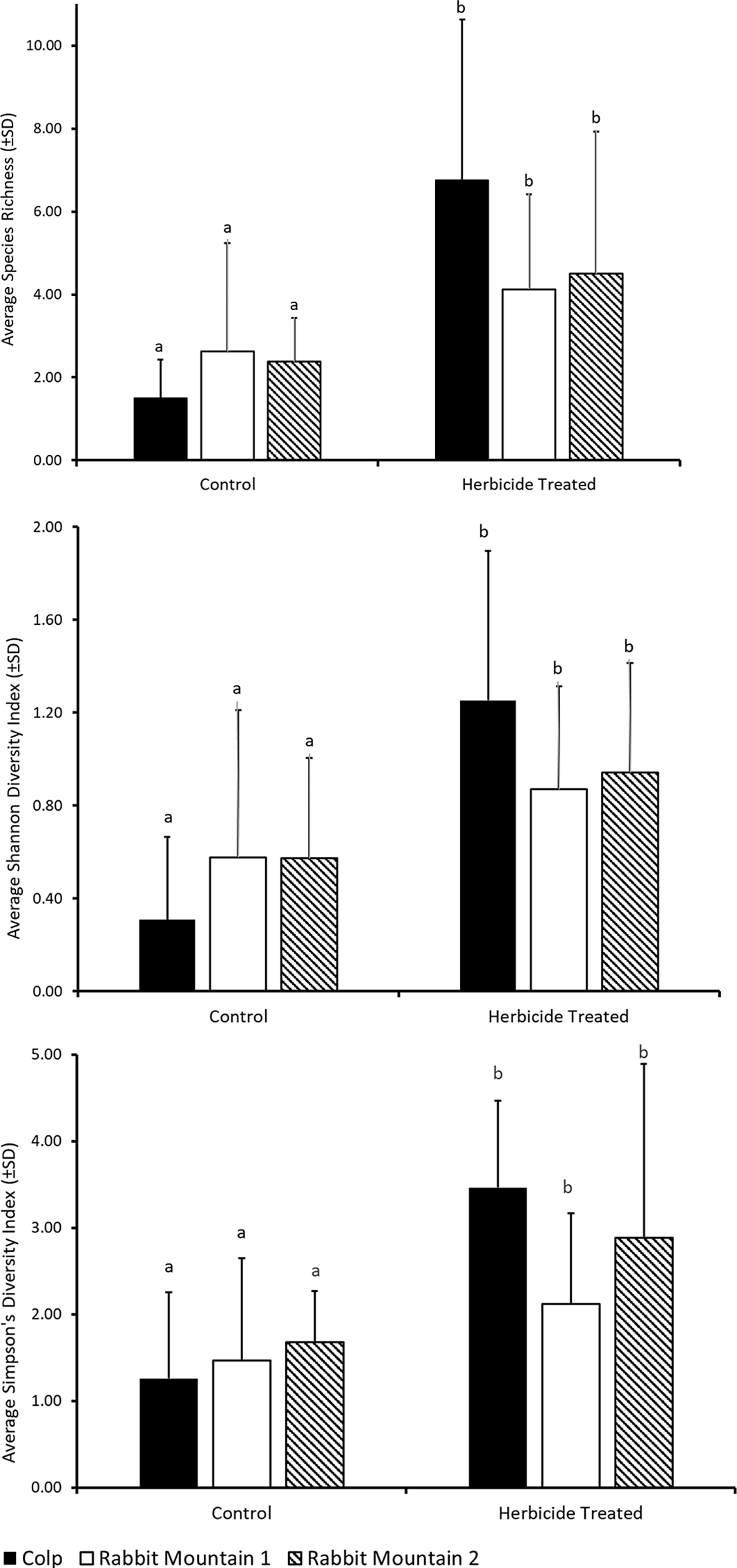
Figure 2. Average (±SD) of floral diversity measures from belt transects. Statistical comparison is across treatments within each location. Different letters indicate significant differences at P < 0.001 using a post hoc Bonferroni comparison (Table 1).
To visualize these diversity measures across seasons, the data were grouped into early (May to early June), mid (June to July), and late (August to September) seasons, as presented in Figures 3 and 4. Table 2 provides the list of pollinator-friendly plant species that were blooming during the study period in the three locations. The impact of herbicide application was consistent in the three locations, suggesting the possibility that previously demonstrated herbicide-mediated control of the invasive grass, B. tectorum (Clark et al. Reference Clark, Sebastian, Nissen and Sebastian2019, Reference Clark, Sebastian, Nissen and Sebastian2020; Sebastian et al. Reference Sebastian, Sebastian, Nissen and Beck2016, 2017a) could be responsible for the growth of pollinator-friendly flora. A noteworthy caveat is that our study did not measure the abundance of B. tectorum in the study plots. Therefore, reduced competitive pressure as a possible means for reestablishment of flowering plant species is a proposed mechanism.

Figure 3. Shannon diversity index values from belt transects for treated and untreated control plots across the season in the different geographic locations.

Figure 4. Simpson’s diversity index values from belt transects for treated and untreated control plots across the season in the different geographic locations.
Table 2. List of flowering pollinator-friendly forb species from random walk sampling in the three geographic locations. a
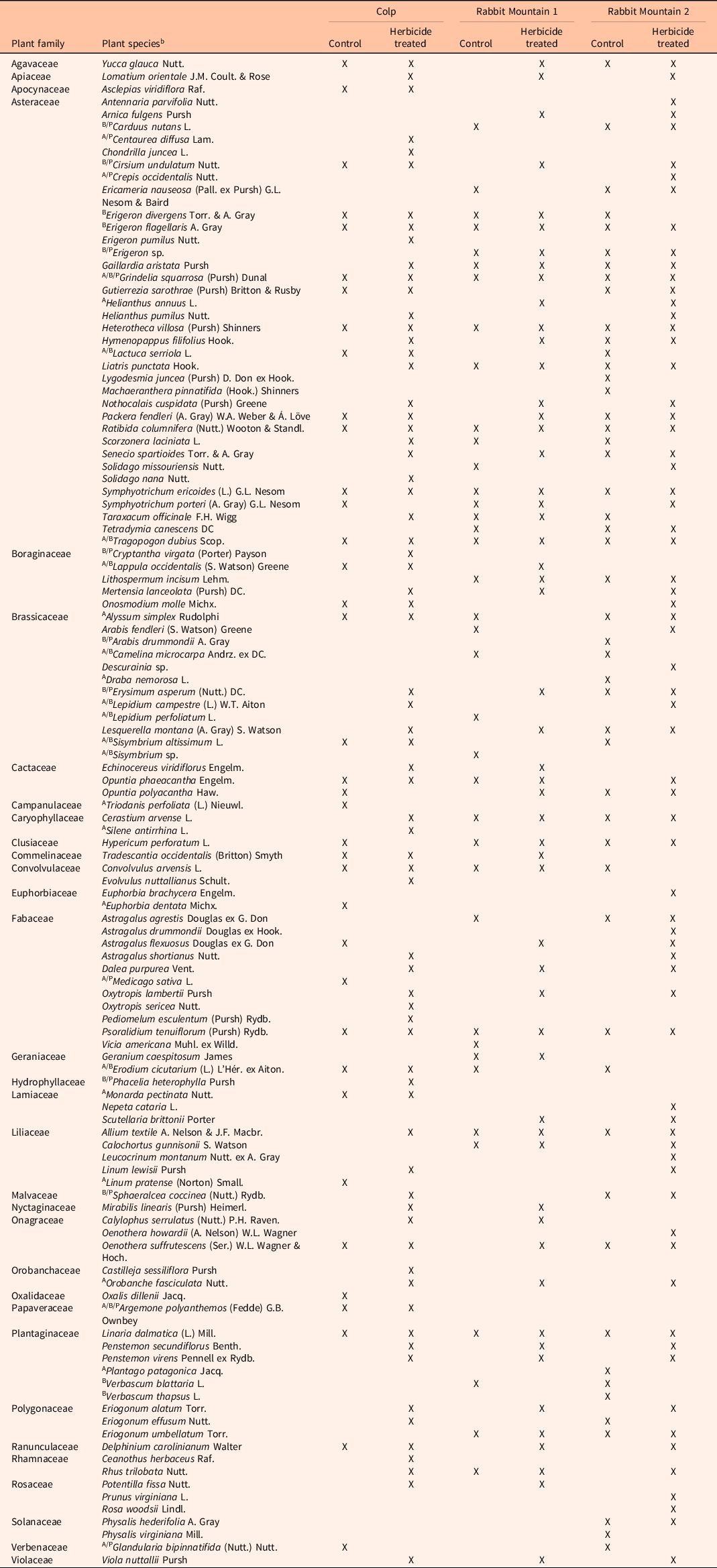
a Plant species are grouped by families. X indicates species seen in the plot during the study period. Only plants that were blooming were recorded in the study. The letters A/B/P preceding the names of some species indicate annual/biennial/perennial life histories, and those without letters preceding their names are all perennials (https://plants.usda.gov/home).
b USDA nomenclature: https://plants.usda.gov/home.
Invasive annual grasses have been shown to impact community composition in ecosystems where they are invasive, leading to potential reductions in abundance and diversity of native species in these ecosystems. However, the intensity of displacement likely depends on the ecological context, specifically the ability of one species to preempt another (Fridley et al. Reference Fridley, Jo, Hulme and Duncan2021; Lenz et al. Reference Lenz, Moyle-Croft and Facelli2003; MacArthur and Levins Reference MacArthur and Levins1967; Pyšek et al. Reference Pyšek, Jarošík, Hulme, Pergl, Hejda, Schaffner and Vilà2012). A decrease in the richness of native species and reduced ecosystem functionality in the presence of invasive species is evident even at smaller spatial scales (Bernard-Verdier and Hulme Reference Bernard-Verdier and Hulme2019). Decrease in species richness has also been previously described in rangelands experiencing B. tectorum invasion (Clark Reference Clark2020; Clark et al. Reference Clark, Sebastian, Nissen and Sebastian2019, Reference Clark, Sebastian, Nissen and Sebastian2020). Our results support this premise that controlling the invasive annual grass B. tectorum can have beneficial impacts on the rangelands by improving the richness and abundance of native flora in the region. It is to be noted that the results of the case study we present is from one flowering season immediately following the winter application of the herbicide.
Ongoing studies on biological invasions and their control suggest that the long-term impact of invasive species removal on native species richness needs further investigation. The benefits of increased species richness and diversity observed soon after control may be modest and may not be long lasting (Adams et al. Reference Adams, Jennings and Warnock2020; Kettenring and Adams Reference Kettenring and Adams2011). In regions experiencing long-term establishment of invasive plant species, it is likely that the diversity of native species in the ecosystem has been compromised (Duncan et al. Reference Duncan, Jachetta, Brown, Carrithers, Clark, Ditomaso, Lym, McDaniel, Renz and Rice2004), though communities with native annual forbs can be impacted (Meyer-Morey et al. Reference Meyer-Morey, Lavin, Mangold, Zabinski and Rew2021). Our study shows that flowering species reappearing in the year following indaziflam application include annuals, biennials, and perennials (Table 2), many of which are native to the region, agreeing with the earlier report that indaziflam application for B. tectorum control does not appear to negatively impact native species richness in the natural areas and rangelands of Colorado (Clark et al. Reference Clark, Sebastian, Nissen and Sebastian2019). It has been suggested that implementing control measures when there is still remaining native vegetation may yield better success in restoration of native species (Davies and Sheley Reference Davies and Sheley2011). Our case study shows reduced flowering plant species diversity in control plots (Figure 2), reiterating the possibility that controlling B. tectorum populations in these rangelands could improve native flowering plant populations. In addition, planning restorative actions needed for assisted reestablishment of native forbs in combination with the application of herbicide for invasive grass control may further promote flowering plant reestablishment.
As mentioned earlier, one limitation of our case study is that we focused on diversity of flowering plants and did not determine the abundance of B. tectorum in the control and treated plots. While there are few studies that explore the direct impacts of invasion by nonnative plant species on pollinators, it is evident that abundance of native flowering species is reduced when ecosystems are dominated by invasive species (Bernard-Verdier and Hulme Reference Bernard-Verdier and Hulme2019). Thus, there is a high likelihood that plants that support the nutritional and nesting needs of pollinators (Blüthgen and Klein Reference Blüthgen and Klein2011; Giannini et al. Reference Giannini, Garibaldi, Acosta, Silva, Maia, Saraiva, Guimarães and Kleinert2015; Soliveres et al. Reference Soliveres, van der Plas, Manning, Prati, Gossner, Renner, Alt, Arndt, Baumgartner, Binkenstein, Birkhofer, Blaser, Blüthgen, Boch and Böhm2016; Tscharntke et al. Reference Tscharntke, Tylianakis, Rand, Didham, Fahrig, Batáry, Bengtsson, Clough, Crist, Dormann, Ewers, Fründ, Holt, Holzschuh and Klein2012) are reduced in such invaded areas. Although this report is a single case study from three locations in the rangelands of Colorado, the immediate benefits of controlling the invasive annual grass B. tectorum are compelling. An earlier study conducted in the same geographic region suggests very little if any residual effects of the herbicide indaziflam (Clark et al. Reference Clark, Sebastian, Nissen and Sebastian2019). However, the nesting biology of the pollinators previously reported in this rangeland ecosystem (Goldstein and Scott Reference Goldstein and Scott2015; Kearns and Oliveras Reference Kearns and Oliveras2009a; Scott et al. 2011) indicates that many of the bee species are ground nesting, wherein the female bees tunnel into the soil, lay eggs, and provision the larvae with pollen that is consumed over the larval developmental period (Buchmann and Nabhan Reference Buchmann and Nabhan1996; Michener Reference Michener1974). It would be critical to determine the extent of herbicide residue in the soil and its potential to impact the development of ground-nesting bee larvae (Buckles and Harmon-Threatt Reference Buckles and Harmon-Threatt2019; Harmon-Threatt Reference Harmon-Threatt2020). Continued monitoring of these locations will help strengthen data on the diversity of native plant species as invasive grasses continue to be controlled. This would also provide critical information on the long-term effectiveness of herbicide use and invasive species control on ecosystem functions.
Acknowledgments
The study design was based on input from Harry Quicke and Steve Sauer. JH and AS would like to thank Nicholas DiMascio and Jim Sebastian for help in the field. The authors received funding from Boulder County Parks and Open Spaces Small Grants and a Bayer vegetation management grant. The authors express their gratitude to two anonymous reviewers and the subject editor whose suggestions greatly improved the quality of the article. The statements made in the article represent authors’ views and should not be interpreted as endorsement from their respective employers or the funding agencies. Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture (USDA). USDA is an equal opportunity provider and employer.








