Introduction
Alpha-lipoic acid (α-LA), also known as lipoic acid (1,2-dithian-3-valeric acid), is a naturally occurring compound in microorganisms, plants and animals. α-LA is lipid-soluble and water-soluble, and therefore, it can be readily absorbed and transported across the cell membrane, thus exerting optimal nutritional effects (Murali and Sherin, Reference Murali and Sherin2020). Because of its chemical properties, it has various biological activities such as scavenging free radicals, antioxidant regeneration and anti-inflammation (Biewenga et al., Reference Biewenga, Haenen and Bast1997; Karafakioğlu, Reference Karafakioğlu2019). α-LA is normally found in mitochondria and is involved in the tricarboxylic acid cycle (Packer and Cadenas, Reference Packer and Cadenas2010) and lipid metabolism (Chen et al., Reference Chen, Kang, Wang and Lee2012) as a cofactor for biological processes. Lipoic acid has been described as a metabolic antioxidant because it is an effective scavenger of reactive oxygen species such as hydroxyl radicals, hypochlorous acid and singlet oxygen species (Bustamante et al., Reference Bustamante, Lodge, Marcocci, Tritschler, Packer and Rihn1998). The oxidized form of lipoic acid also effectively scavenges free radicals while enhancing the activity of antioxidant enzymes (Srilatha et al., Reference Srilatha, Redely, Qudratullah and Raju2010). Lipoic acid may also be used as an anti-inflammatory agent in the treatment of a variety of diseases (Bilska and Wlodek, Reference Bilska and Wlodek2005; Konrad, Reference Konrad2005; Gorąca et al., Reference Gorąca, Huk-Kolega, Piechota, Kleniewska, Ciejka and Skibska2011).
α-LA has been widely used in the pharmaceutical and nutritional health care industries (Dos Santos et al., Reference Dos Santos, Romeiro, Rodrigues, Cerqueira, Monteiro, João and Barreira2019). In addition, α-LA has also been partially studied in the practical application of ruminants such as sheep (Luo et al., Reference Luo, Ju, Chang, Ge, Zhao and Zhang2022; Yang et al., Reference Yang, Zhang, Pang, Zhang, Fu, Wang, Liu and Gao2023), goats (Wang et al., Reference Wang, Zhou, Zhou, Hou and Shi2017), dairy cows (Fiore et al., Reference Fiore, Perillo, Piccione, Gianesella, Bedin, Armato, Giudice and Morgante2016; Luo et al., Reference Luo, Ju, Chang, Ge, Zhao and Zhang2022) and beef cattle (Schmidt et al., Reference Schmidt, Olson, Meyer, Brandt, Rentfrow, Stahl and Berg2005), and the results showed that α-LA could promote animal growth, improve meat quality and enhance antioxidant capacity. Because α-LA synthesized by the animal body cannot meet the needs, it needs to be supplemented from the diet. Low doses of synthetic α-LA have been shown to be safe and nontoxic as a dietary supplement for herbivores (Xu et al., Reference Xu, Wang, Han, Qi, Li, Guo, Qin and Chen2019). The safe single oral dose of α-LA in dairy cattle is approximately 77–100 mg/kg body weight and the lethal dose is approximately 154–200 mg/kg body weight (Luo et al., Reference Luo, Ju, Chang, Ge, Zhao and Zhang2022). At present, there are few studies on the effect of α-LA on nutrient metabolism in dairy cows. The aim of this study was to investigate the effects of dietary supplementation of α-LA at doses lower than safe oral doses on performance, immunity, anti-oxidation and serum metabolism in Holstein dairy cows, providing a theoretical basis for the basic study of α-LA as a feed additive in nutritional metabolism of livestock and poultry.
Materials and procedures
Animals and feeding management
Henan Agricultural University Animal Care and Use Committee approved the experimental protocol (Permit No. HNND2019031018). A total of 30 multiparous lactating Holstein cows (2–3 parity, 550 ± 25 kg, 200 ± 15 days lactation) were randomized to three treatment groups: CTL group (control group), LA-L group (low-dose of α-LA group) and LA-H group (high-dose of α-LA group), and the addition of α-LA was 0, 30 and 60 mg/kg per head per day, respectively. The dosage of α-LA has been based on the body weight (BW) and α-LA was sprinkled on the total mixed ration (TMR). 7 days prefeeding period, 30 days formal period. All cows were fed TMR (Table 1), and had free access to water and exercise. Daily feeding and remaining TMR were weighed and recorded for each cow. The daily milk production of each cow was recorded using a milk sampling device (Waikato Milking Systems NZ Ltd., Waikato, Hamilton, New Zealand), and the average daily milk production of each group was calculated.
Table 1. Ingredients and chemical composition of diet (g/kg of DM)
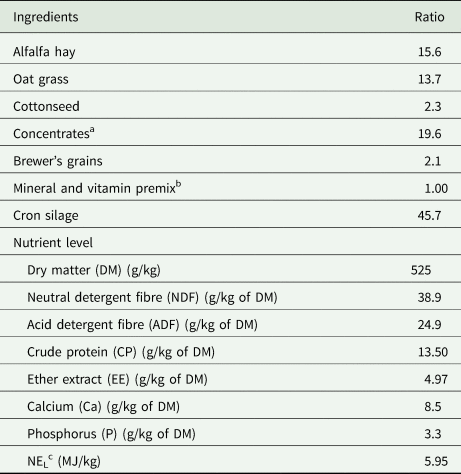
a Composition of concentrate supplementary nutrition (g/kg of DM): DM (886.8 g/kg), NDF (273.7 g/kg), ADF (105.8 g/kg), CP (190.7 g/kg), EE (60.8 g/kg), Ca (19.3 g/kg), P (6.3 g/kg), gross energy (GE, 174.2 MJ/kg).
b Premix contained 0.32 g of Co/kg, 13.3 g of Cu/kg, 0.5 g of I/kg, 0.04 g of Fe/kg, 33.4 g of Mn/kg, 8 g of Se/kg, 56.2 g of Zn/kg, 1 800 000 IU of vitamin A/kg, 200 000 IU of vitamin D/kg, and 15 000 IU of vitamin E/kg.
c NEL was a calculated value (CPM-Dairy software 3.0.8.1), while the others were measured values.
Sample collection
Last day of the trail, milk composition and somatic cell count (Delta, CombiScope FTIR 600, Netherlands) were measured by collecting milk samples (morning, midday and evening milking) with bronopol tablets in a 4:3:3 ratio and storing them at 4°C (Laporte and Paquin, Reference Laporte and Paquin1999). Blood samples were collected using 10 ml heparin-sodium anticoagulation vacuum tubes and coagulant vacuum tubes via the coccygeal vessels prior to morning feeding. Vacuum tubes containing blood were immediately placed on ice and transported to the laboratory. Plasma and serum were extracted by centrifugation at 1500 × g for 10 min at 4°C, then transferred to Eppendorf tubes (1.5 ml) and stored at −80°C until further analysis.
Chemical analysis
Dry matter (DM) content of diet samples was analysed and evaluated after oven drying at 105°C for 24 h (Latimer, Reference Latimer2016). The formula N × 6.25 was used to calculate crude protein (CP) (Latimer, Reference Latimer2016). Ether extract (EE) was measured using a Soxtec instrument (Tecator) according to the method of AOAC (Latimer, Reference Latimer2016). Dietary neutral detergent fibre (NDF) and acid detergent fibre (ADF) were measured with a fibre analyser (Ankom 2000i; ANKOM Technology, Macedon, NY, USA) according to (VanderZaag et al., Reference Vanderzaag, Wagner-Riddle, Park and Gordon2011). Calcium (Ca) was measured using disodium edetate (neq ISO: 1987). Phosphorus (P) was determined using a spectrophotometer (TU-1810 UV-Spectrophotometer, Beijing Purkinje General Instrument Co., Ltd. China) according to AOAC regulations (Latimer, Reference Latimer2016).
Biochemical parameters, including alanine aminotransferase (ALT), aspartate aminotransferase (AST), bilirubin (TBIL), urea (UREA), glucose (GLU), cholesterol (CHOL), low-density lipoprotein (LDL), very low-density lipoprotein (VLDL), high-density lipoprotein (HDL), triglyceride (TG), total protein (TP), albumin (ALB) and globulin (GLO), were measured using an automatic biochemical analyser (Hitachi 7600). Other blood parameters included antioxidant parameters: nitric oxide synthase (NOS), total superoxide dismutase (T-SOD), glutathione reductase (GR) and malondialdehyde (MDA); hormonal parameters: growth hormone (GH), cortisol (CORT), triiodothyronine (T 3), glucagon (GC), insulin (INS) and insulin-like growth factor-I (IGF-I); immune parameters: immunoglobulin A (IgA), IgG, tumour necrosis factor-α (TNF-α), interleukin-1β (IL-1β), IL-2, IL-8, IL-10, IL-12, interferon γ (IFN-γ) and heat-shock protein 70 (HSP70) were measured spectrophotometrically using ELISA kits from (Shanghai Blue Gene Biotechnology Co., Ltd) following the manufacturer's instructions. It should be noted that plasma was used for detecting hormones and serum for other biochemicals. It should be noted that plasma was used for detecting hormones and serum for other biochemicals.
Metabolomics determination
Serum was thawed on ice at 4°C and 100 μl was transferred to a 1.5 ml EP tube. Extraction liquid (V methanol:V acetonitrile:VH2OH2O = 2:2:1) (350 μl) and L-2-chlorophenylalanine (20 μl) were added and vortexed for 30 s. Sonication for 10 min, storage at −20°C for 1 h and centrifugation at 12 000 rpm for 15 min at 4°C. Pipet 0.4 ml of the supernatant into a new 1.5 ml EP tube and dry in a vacuum concentrator. After drying, 100 μl of extraction fluid (V acetonitrile:V water = 1:1) was added for recombination, vortexed for 30 s, sonicated in a water bath at 4°C for 10 min, and then centrifuged at 12 000 rpm for 15 min at 4°C. 10 μl of the supernatant was pipetted from each sample and pooled together as a QC sample. Supernatants and QC samples (60 μl) were analysed by ultra performance liquid chromatography (UHPLC) quadrupole time of flight (QTOF) mass spectrometry (MS).
LC-MS/MS analysis was performed on a UHPLC BEH amide (1.7 μm × 2.1 mm × 100 mm, Waters). The injection volume was 2 μl and the source temperature was set at 55°C. The mobile phase was composed of phases A (containing 25 mM NH4OAc and 25 mM aqueous solutions, pH = 9.75) and B (acetonitrile). The mobile phase was eluded with the following gradient: 0 min, 1% A; 1 min, 1% A; 8 min, 100% A; 10 min, 100% A; 10.1 min, 1% A; 12 min, 1% A, at a flow rate of 0.3 ml/min. MS/MS spectra were acquired during the LC/MS experiment. Electrospray ionization (ESI) source conditions: ion source gas 1 = 60 Pa; ion source gas 2 = 60 Pa, curtain gas = 30 Pa; source temp = 550°C; ion spray voltage floating = 5500 V (positive operation) or − 4500 V (negative operation).
Statistical analysis
The MS data were converted to mzXML format using MS Converter and processed using R package XCMS (v. 1.41.0) (Smith et al., Reference Smith, Want, O'Maille, Abagyan and Siuzdak2006). The resulting 3D data (sample names, peak counts and normalized peak areas) were entered into the SIMCA 14.1 software package (MKS Data Analytics Solutions, Umea, Sweden) (Dunn et al., Reference Dunn, Broadhurst, Begley, Zelena, Francis-Mcintyre, Anderson, Brown, Knowles, Halsall and Haselden2011) for principal component analysis (PCA; Elhaik, Reference Elhaik2022) and orthogonal partial least square discriminant analysis (OPLS-DA; Wiklund et al., Reference Wiklund, Johansson, Sjöström, Mellerowicz, Edlund, Shockcor, Gottfries, Moritz and Trygg2008). A metabolite was considered to be significantly if its VIP > 1 and P value < 0.05. For differential metabolites, impact value ≥ 0.1 or P value ≤ 0.05 as significance threshold, through the KEGG online database (http://www.genome.jp/kegg/) and the Bos taurus (cow) pathway database genome profiles to identify major enriched pathways.
SPSS 19.0 was used to analyse data on feed intake, milk production and quality, blood physiological, metabolic and oxidative responses. Tukey multiple range test was used to compare significant mean differences using the criteria of trend 0.05 < P < 0.1, significance P ≤ 0.05, and highly significance P ≤ 0.01.
Results
Effects of α-LA on feed intake, milk production and milk component
As shown in Table 2, milk yield was higher (P < 0.05) after supplementation with α-LA, the somatic cell counts in LA-L group were significantly lower (P < 0.05) than those in CTL group, and the feed intake, milk lactose, protein, fat and total milk solids levels were not different (P > 0.05). From Fig. 1, cows supplemented with α-LA from day 8 had a higher average daily milk yield than the CTL group.
Table 2. Effects of α-LA on feed intake, milk yield and quality in dairy cows (n = 10)

a α-LA was added to a basal diet at 0 (CTL), 30 (LA-L), 60 (LA-H) mg/kg of BW per day for each cow.
b s.e.m., standard error of the mean.

Figure 1. Effect of alpha-lipoic acid (α-LA) on average daily milk yield of dairy cows.
Effect of α-LA addition on serum biochemical and antioxidant indices in Holstein cows
The routine biochemical indices and antioxidant parameters (Table 3), as shown in Table 3, the levels of HDL and TG were significantly higher (P < 0.05) after α-LA supplementation; regarding antioxidant parameters, the levels of NOS and GR in the LA-L group were significantly lower (P < 0.05) than those in CTL group.
Table 3. Serum biochemical and antioxidant indices (n = 10)
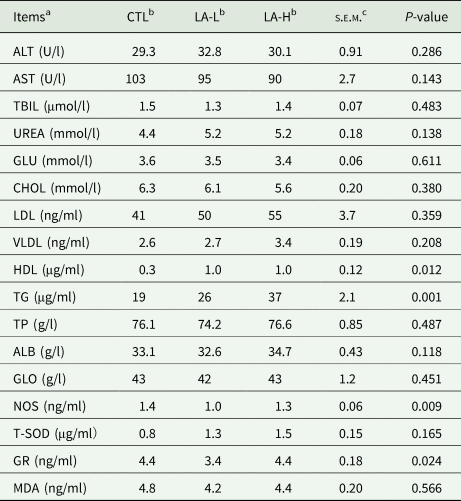
a ALT, alanine aminotransferase; AST, aspartate aminotransferase; TBIL, bilirubin; UREA, urea; GLU, glucose; CHOL, cholesterol; LDL, low-density lipoprotein; VLDL, very low-density lipoprotein; HDL, high-density lipoprotein; TG, triglyceride; TP, total protein; ALB, albumin; GLO, globulin; GR, glutathione reductase; MDA, malondialdehyde; NOS, nitric oxide synthase; T-SOD, superoxide dismutase.
b α-LA was added to a basal diet at 0 (CTL), 30 (LA-L), 60 (LA-H) mg/kg of BW per day for each cow.
c s.e.m., standard error of the mean.
Effect of adding α-LA on nutrition-related metabolic hormones in plasma of Holstein cows
Hormones associated with nutrition-related metabolism in plasma are shown in Table 4. The levels of INS tendency to decrease (0.05 < P < 0.10) after α-LA supplementation, and the concentrations of CORT and T 3 in the LA-H group were significantly higher (P < 0.05) than those in CTL group.
Table 4. Plasma levels of metabolic hormones in Holstein cows (n = 10)
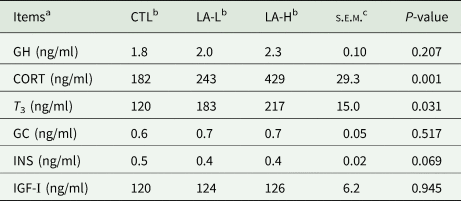
a CORT, cortisol; GC, glucagon; GH, growth hormone; INS, insulin; IGF-Ⅰ, insulin-like growth factor-I; T 3, triiodothyronine.
b α-LA was added to a basal diet at 0 (CTL) mg/kg, 30 (LA-L) mg/kg, 60 (LA-H) mg/kg BW per day for each cow.
c s.e.m., standard error of the mean.
Effect of adding α-LA on serum immune indexes in Holstein cows
As shown in Table 5, the levels of IgG and IgA tended to increase (0.05 < P < 0.10) after α-LA supplementation, the levels of inflammation-related factors such as TNF-α and IL-2 were significantly higher in the LA-L group than in the CTL group, and the levels of IL-1β and HSP70 were significantly higher (P < 0.05) in the LA-Hs than in the CTL group.
Table 5. Serum levels of immune-related indices in Holstein cows (n = 10)
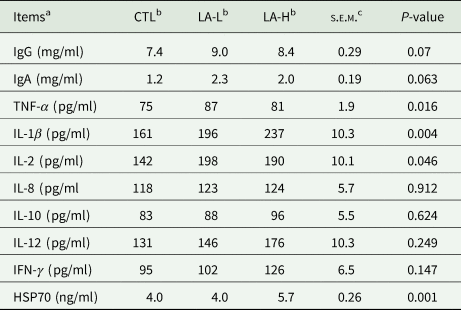
a IFN-γ, interferon-γ; IL, interleukin; TNF-α, tumour necrosis factor α; Ig, immunoglobulin; HSP70, heat-shock protein 70.
b α-LA was added to a basal diet at 0 (CTL) mg/kg, 30 (LA-L) mg/kg, 60 (LA-H) mg/kg BW per day for each cow.
c s.e.m., standard error of the mean.
LC–MS analyses of metabolic profiles
The screening yields in positive and negative ion mode were 83.94% and 87.92%, respectively, under the conditions of pooled QC sample relative standard deviation (<30%) and feature yield (>80%), indicating that the method has good stability and repeatability. In Fig. 2a, the PCA score plots no clear difference was observed between the CTL group, LA-L group and LA-H groups. This suggests that changes in endogenous small serum metabolites were not evident between the groups. Figures 2b and 2c show the OPLS-DA results for both positive and negative ion modes. A cluster of distinct separations was achieved between any two sets of metabolite profiles.

Figure 2. Scatter plot of the principal component analysis (PCA) and the orthogonal partial least-square discriminant analysis (OPLS-DA).
Table 6 summarizes the RT, VIP, m/z, fold change (FC), and P values for each metabolite. In the positive ion mode, 5, 5 and 4 different metabolites were identified between groups, respectively. Similarly, 7, 5 and 5 different metabolites were identified between groups in the negative ion mode. Taking into account the overlapping of pairs of metabolites, a total of 13 and 15 metabolites differed in the positive/negative ion mode across the three experimental groups.
Table 6. Differentially expressed endogenous metabolites between groups
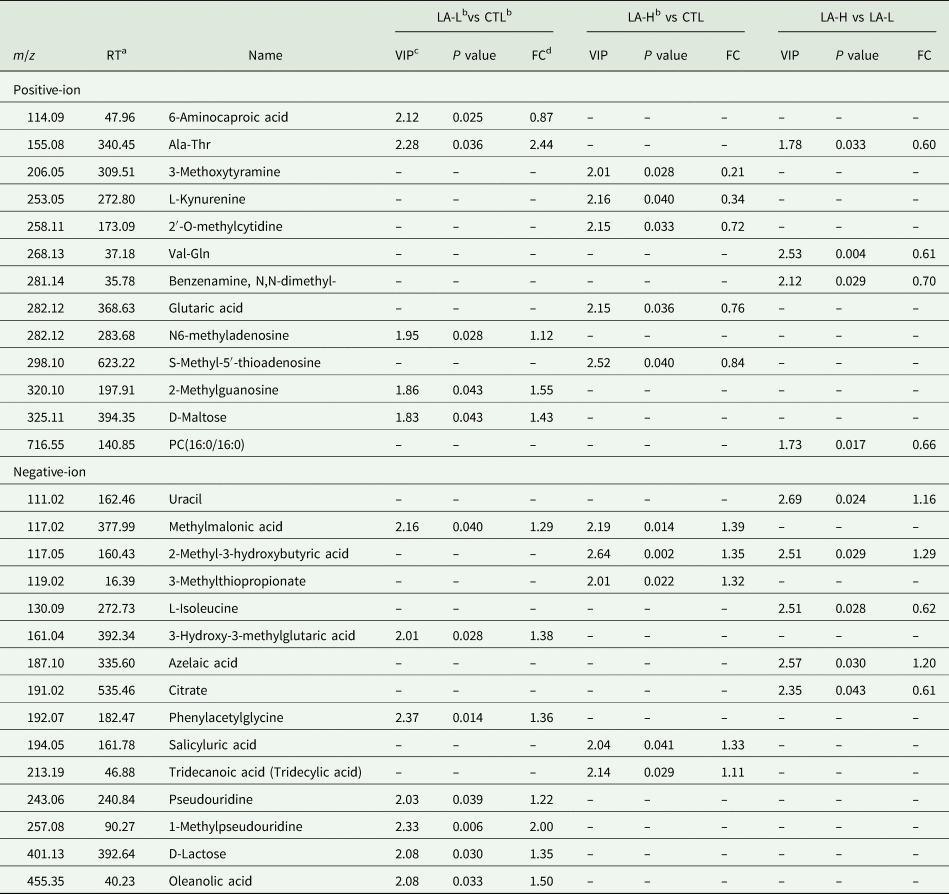
a RT means retention time.
b α-LA was added to a basal diet at 0 (CTL), 30 (LA-L), 60 (LA-H) mg/kg of BW per day for each cow.
c VIP means variable importance in the projection.
d FC (fold change) was calculated by the average value of the two compared groups. The screening conditions for differential metabolites were VIP > 1 and P value < 0.05; if FC > 1, it means the concentration of the metabolites are more.
Key metabolic pathway
Table 7 shows the 13 affected metabolic pathways. Based on the criteria of impact value ≥ 0.1 or P value < 0.05, a total of four major impact metabolic pathways were identified in the LA-H group v. LA-L group, including the glyoxylate and dicarboxylate metabolic pathways (impact value = 0.30, Raw P = 0.03), pantothenate and CoA biosynthesis (impact value = 0.00, Raw P = 0.03), beta-alanine metabolism (impact value = 0.00, Raw P = 0.04) and citric acid cycle (TCA cycle) (impact value = 0.05, Raw P = 0.04), and the metabolites from the four metabolic pathways were citric acid and uracil. Starch and sucrose metabolism were also differentially involved between CTL group and LA-L group, which exhibited the lowest Raw P = 0.02 from the pathway topology analysis (impact value = 0.07), and the main affected metabolite was D-maltose.
Table 7. Altered metabolic pathways with metabolomics pathway analysis (MetPA)
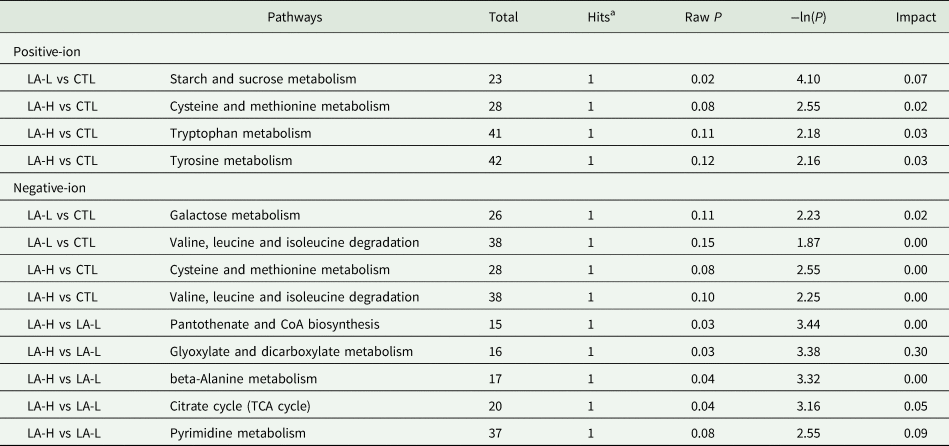
a Hits: the number of the metabolites mapped to their related pathway. Raw P: the original P value calculated from the enrichment analysis. Impact: impact value of topological analysis of metabolic pathways.
Discussion
Effect of dietary supplementation of α-LA on nutritional metabolism in dairy cows
Metabolic hormones associated with nutrient metabolism (T 3, GH, GC, INS and CORT) are commonly used as important indicators of stress intensity in vivo (Sejian et al., Reference Sejian, Bagath, Krishnan, Rashamol, Pragna, Devaraj and Bhatta2019). CORT is synthesized by the adrenal cortex and regulates behavioural and neuroendocrine activity in animals (Krishnan et al., Reference Krishnan, Silpa, Sejian, Das, Sejian, Mukherjee and Banerjee2023). CORT has been found to bind to specific receptors in mammary tissue and is involved in the regulation of milk protein secretion (Kay, Reference Kay2020). For early lactating cows, CORT stimulates glucose production and helps to meet the high glucose demand of early lactating cows due to increased milk production (Gross et al., Reference Gross, Wellnitz and Bruckmaier2015). Bomfim showed (Bomfim et al., Reference Bomfim, Merighe, de Oliveira and Negrao2022) that increased CORT secretion increased milk production in goats. In addition, some in vivo studies on sheep have shown that increased cortisol secretion leads to increased somatic cell counts in milk (Mehdid et al., Reference Mehdid, Martí-De Olives, Fernández, Rodríguez and Peris2019; Hooper et al., Reference Hooper, Dos, de Oliveira, Merighe, Titto and Negrao2021). The results of this experiment aligned with the aforementioned effects of CORT; following α-LA supplementation, the plasma CORT levels in the LA-H group was significantly higher compared to the CTL group, the lactation volume of dairy cows increased, and the somatic cell count in milk increased. T 3 regulates the metabolism of substances and energy in the body and mainly promotes the oxidative decomposition of energy substances such as protein, glucose and fat (Gu et al., Reference Gu, Yang, Gong, Ma, Yan, Huang, Wang and Peng2021). Prolactin (PRL) is regulated by T 3 through the hypothalamic–pituitary–adrenal axis, and T 3 levels are positively correlated with PRL levels (Bibi et al., Reference Bibi, Shah, Malik and Goosens2021). Serum T 3 levels were significantly higher in the LA-H group than in the CTL group in this study. Thus, dietary supplementation of α-LA increases metabolism in lactating dairy cows.
HDL and LDL reflect the breakdown and transport of lipids in animals (Wang et al., Reference Wang, Yang, Tan, Xiao, Jia, Dong, Chi, Liu and Zhang2018a). HDL is converted and metabolized by the liver by transporting CHOL from animals back to the liver in the form of high HDL-CHOL (Kondo and Watabe, Reference Kondo and Watabe2006). The content of TG in serum reflects the metabolic status of the body, HDL and LDL are carrier proteins of lipids, and the change of their content is positively correlated with the change of TG. The higher the HDL, the greater the body's ability to transport CHOL back to the liver (Wang et al., Reference Wang, Wang, Huang, Li, Sun, Wang and Ma2018b; Li et al., Reference Li, Yan, Dong, Pan, Tan, Zhang, Suo, Li, Huang, Yang, Zhang and Li2022). In this experiment, HDL and TG levels in blood were significantly increased after dietary supplementation of α-LA, and CHOL levels gradually decreased with increasing HDL, while TG was the main form of energy storage in dairy cows. Thus, dietary supplementation of α-LA can provide more energy to the body by improving lipid metabolism. In addition, α-LA and its reduced form DHLA enhance cellular glucose uptake through insulin stimulation (Rhoads et al., Reference Rhoads, Baumgard, Suagee and Sanders2013). In this study, dietary supplementation of α-LA significantly increased milk yield while decreasing INS levels, therefore, the results of this experiment are consistent with previous findings and inferences.
According to metabolomics analysis, LA mainly affected the metabolism of fatty acids, amino acids and glucose in lactating dairy cows. Elevated levels of methylmalonic acid (MMA) in serum represent significant metabolic changes associated with vitamin B12 (VB12), and serum MMA concentration is the preferred indicator for judging functional VB12 deficiency (Gültepe et al., Reference Gültepe, Özcan, Avşar, Cetin, Özdemir and Gök2003). Therefore, appropriate VB12 supplementation is recommended when α-LA is included in the diet. Therefore, appropriate supplementation of VB12 is recommended when α-LA is included in the diet. Additionally, the levels of D-maltose and D-lactose were higher in the LA-L group compared to the CTL group, which might contribute to the increased milk yield observed in the LA-L group. Oleanolic acid has the function of enhancing the effect of insulin and improving the immune capacity of animals (Castellano et al., Reference Castellano, Ramos-Romero and Perona2022). These results indicate that dietary supplementation of 30 mg/kg BW α-LA is beneficial for lipid metabolism and immune competence in dairy cows. Analysis of the affected metabolic pathways showed that the major metabolites were citric acid and uracil and D-maltose. Among these, pantothenic acid and metabolites from the CoA biosynthesis pathway, despite not being present as metabolites themselves, are integral components of coenzyme A accessory groups and acyl carrier proteins (Lanska, Reference Lanska2012). They play a crucial role in carbohydrate, protein and lipid metabolism (Wang et al., Reference Wang, Zhang, Yue, Ge, Zhang, Ma and Kong2016). Additionally, pantothenic acid promotes glutathione biosynthesis, thereby reducing apoptosis and cellular damage (Abdelqader and Al-Fataftah, Reference Abdelqader and Al-Fataftah2016). Consequently, this may explain why multiple differential metabolites and metabolic pathways exist.
Effects of dietary supplementation of α-LA on the antioxidation in dairy cows
GR and SOD are important antioxidants, and their activities directly reflect the antioxidant capacity of the body (Zhang et al., Reference Zhang, Yang, Li, Wang, Fu, Li and Gao2021). MDA is a product of lipid peroxide and oxidative stress in vivo and reflects the extent of lipid peroxidation in tissue cells (Gong and Xiao, Reference Gong and Xiao2016). Therefore, MDA is another important indicator of antioxidant capacity in tissue cells. NOS is the catalytic enzyme that generates the nitric oxide (NO) reaction, and although NO has a very short half-life, it can rapidly react with oxygen free radicals in vivo, thereby reducing free radicals (Król and Kepinska, Reference Król and Kepinska2020). In this study, NOS and GR levels in the LA-L group were significantly lower than those in the CTL group, but there was no significant difference between the LA-H group and the CTL group, indicating that α-LA had a significant effect on the antioxidant system in dairy cows at a dose of 30 mg/kg. Following LA supplementation, there was a trend of increasing SOD levels and decreasing MDA levels. This suggests that LA enhances the antioxidant capacity of dairy cows, inhibits or removes reactive oxygen species (ROS) generated by lipid peroxidation and oxidative stress, and thus protects or mitigates the damage caused by oxidative stress to the body, tissues and cells.
Effects of dietary supplementation of α-LA on the immunity in dairy cows
HSP70 is a family of proteins known as cell thermometers due to its potential role in thermotolerance (Mishra and Palai, Reference Mishra and Palai2014). HSP70 functions as a molecular chaperone and plays an important role in cellular thermotolerance, apoptosis and immune regulation (Vasaikar et al., Reference Vasaikar, Ghosh, Narain, Basu and Gomes2015). Heat-shock proteins can activate immunity by linking Toll-like receptors (TLRs) (Takeda and Akira, Reference Takeda and Akira2003), which HSP70 promotes dendritic cell proliferation by stimulating TLR4 (Fang et al., Reference Fang, Wu, Huang, Wang, Ang, Cao and Wan2011). TLR4 recognizes damage-associated molecular patterns, thereby many proinflammatory cytokines and triggers host immune responses (Kawai and Akira, Reference Kawai and Akira2010). Extracellular heat-shock proteins are positively associated with increased innate immune responses (Calderwood et al., Reference Calderwood, Gong and Murshid2016). Therefore, HSPs play an important role in enhancing immunity. In addition, HSP70 enters cells through the mediation of cytokines such as CD40 and Toll-like receptors (TLRs), thereby activating the myeloid differentiation factor 88/NF-κB signalling pathway, which in turn synthesizes and releases more pro-inflammatory factors such as TNF-α and IL-1β to regulate body immune function (Henderson, Reference Henderson2010). In this study, HSP70 content in LA-H group was significantly higher than CTL and LA-L groups, indicating that α-LA supplementation enhanced immune competence in dairy cows. However, the levels of IL-1β and TNF-α increased in the test group, which may be an inflammatory response stimulated by α-LA in lactating cows.
Conclusions
Adding α-LA to the diet of Holstein cows could enhance milk production by improving antioxidant capacity and immunity. Meanwhile, α-LA increased the material circulation and energy flow of dairy cows by regulating biochemical reactions, metabolism-related hormones and pathways. At the same time, it was found that α-LA supplementation could increase the content of MMA in serum, which suggested that VB12 should be added when supplementing feed with α-LA. According to all indexes, adding 30 mg/kg α-LA based on body weight to the diet of dairy cows was more beneficial for dairy cows. These findings could provide valuable data and a firm theoretical foundation for the application of α-LA supplementation in dairy cows. Nonetheless, whether dietary supplementation of α-LA results in additional benefits and long-term effects in dairy cows will require further studies to investigate.
Acknowledgements
The authors would like to thank Shanghai Biotree Biotech Co., Ltd. for their technical support. This study was supported by a grant from the China Agriculture Research System (CARS-36).
Author contributions
Hongrui Zhang, Hongxia Lian and Tengyun Gao conceived and designed research. Gaiying Li and Tong Fu contributed new reagents or analytical tools. Hongrui Zhang and Liyang Zhang conducted experiments. Gaiying Li and Linfeng Wang analysed data. Hongrui Zhang wrote the manuscript. Zhiguo Jiang revised the manuscript and submitted the final version of the manuscript. All authors read and approved the manuscript.
Funding statement
This study was supported by a grant from the China Agriculture Research System (CARS-36).
Competing interests
The authors declare no conflicts of interests.
Ethical standards
All procedures in this study were performed in accordance with the legislation of China regarding the use and care of laboratory animals. Henan Agricultural University Animal Care and Use Committee approved the experimental protocol (Permit No. HNND2019031018).












