Introduction
Clinical and subclinical enteric diseases are associated with significant economic losses to the global poultry industry. Coccidiosis and necrotic enteritis (NE) are two enteric poultry diseases which often occur concurrently in commercial production (Williams, Reference Williams2005). Subclinical coccidiosis negatively impacts gut structures, feed utilisation, and overall growth performance. There are approximately six Eimeria spp. associated with commercial poultry including E. acervulina, maxima and tenella. Eimeria spp. are intracellular parasites which infect intestinal epithelial cells, which is associated with epithelial lesion formation and villi atrophy. Such gut damage has been identified as a predisposing factor to NE (Williams Reference Williams2005). It has been estimated that NE affects up to 40% of commercial broiler flocks and has been estimated to cost the industry approximately 5¢ per broiler in the United States alone (McDevitt et al., Reference McDevitt, Brooker, Acamovic and Sparks2006) and contributes to annual loss of more than $3 billion worldwide (Williams, Reference Williams1999). Such loss is mostly associated with subclinical disease resulting in poor growth performance and feed efficiency. Anti-coccidial products (ionophores and synthetic compounds) are routinely used as preventative measures, however resistance to anti-coccidial treatments in field isolates have been documented (Peek and Landman, Reference Peek and Landman2003). With the increased push towards antibiotic-free poultry production in the U.S.A. and the ban of antibiotic-growth promoters (AGP) in the EU, the pressure to find alternative products to control these two economically detrimental enteric diseases is growing.
Bacillus-based direct-fed microbials (DFM) have gained increased attention as alternatives to AGP use and can support broiler growth performance (Amerah et al., Reference Amerah, Quiles, Medel, Sánchezc, Lehtinend and Gracia2013; Dalloul et al., Reference Dalloul, Lillehoj, Shellem and Doerr2003; Lee et al., Reference Lee, Lillehoj and Siragusa2010a). Furthermore, previous work reported that these DFM reduced clinical signs of experimental avian coccidiosis and increased protective immunological parameters in broiler chickens (Lee et al., Reference Lee, Lee, Lillehoj, Li, Jang, Babu, Park, Kim, Lillehoj, Neumann, Rehberger and Siragusa2010b; Waititu et al., Reference Waititu, Yitbarek, Matini, Echeverry, Kiarie, Rodriguez-Lecompte and Nyachoti2014).
The increasing cost of commercial feed ingredients has forced producers to use cheaper by-products (such as Distiller's dried grains with solubles (DDGS) and wheat middlings) often containing high levels of anti-nutritional factors such as non-starch polysaccharides (NSP). NSP can increase water holding capacity and intestinal viscosity thereby reducing nutrient digestibility. Consequently, feed enzymes have been increasingly used in animal feed to increase the nutrient availability and utilisation of ingredients. Carbohydrases and proteases are two enzymes that improve the nutritional value of feeds and have been shown to confer potentially beneficial changes in intestinal microbial populations (Kiarie et al., Reference Kiarie, Romero and Nyachoti2013). Previous investigations suggested that xylanase, amylase and protease (XAP) and DFM can act synergistically to improve feed utilisation, gut health and bird performance, particularly under challenge conditions (Mathis et al., Reference Mathis, Hofacre, Romero and Lumokins2013; Dersjant-Li et al., Reference Dersjant-Li, Romero, Wealleans and Awati2014).
Most studies to date have been performed in research settings that lack many of the environmental challenges found in commercial facilities. The objective of the current study was to assess the effect of XAP + DFM combination in birds with a mild coccidial challenge on growth performance, intestinal morphology and immune responses of broilers raised on built up litter to simulate commercial production conditions.
Materials and methods
All experimental procedures were reviewed and approved by the University of California Davis Animal Care and Use Review Committee.
Animals and facility
One-day old, male Cobb 500 broiler chicks were obtained from Foster Farms hatchery; Waterford CA. Chicks were vaccinated at the hatchery for Marek's disease virus. Chicks (n = 192) were randomly placed in pens of three brooder-batteries with raised wire floors (Petersime Inc. Gettysburg, OH). The initial brooder temperature was 36°C and was adjusted daily as recommended by the Cobb broiler management guide. The brooders were housed in a climate controlled room with a temperature of 25°C and a relative humidity of 38%. Prior to use of the brooders for this experiment, three cycles of chick brooding had occurred without clean-out between batches. This resulted in the build-up of litter, faeces, dust, and dander under the raised wire floors prior to the initiation of this experiment, mimicking conditions within commercial houses. The lighting schedule was 18L:6D. There were no significant differences in bird body weights between pens. For the duration of the study from day 1–21 days of age, birds had ad libitum access to the experimental mash diets and fresh water.
Experimental design and test diets
Four dietary treatments were tested in a 2 × 2 factorial arrangement in a completely randomised design. This included two levels of challenge (without or with a coccidia challenge), two levels of feed additive (with or without XAP plus DFM). The enzyme blend provided 2000 U/kg of an endo-xylanase from Trichoderma reesei, 200 U/kg of alpha-amylase from Bacillus licheniformis and 4000 U/kg of a serine protease from B. subtilis (XAP; Danisco Animal Nutrition/DuPont). The DFM contained a combination of spores from three strains of Bacillus at 75000 CFU/g (Danisco Animal Nutrition/DuPont). Treatments were: 1) Unchallenged control (UC), 2) UC + XAP + DFM, 3) coccidia challenged control (CC), 4) CC + XAP + DFM. Eight replicate pens containing six chicks each were assigned to each of the four treatments. At five days of age all birds in the challenged groups were oral gavaged with a six-fold concentration of Advent® coccidian vaccine, which contained live oocysts of Eimeria acervulina, Eimeria maxima, Eimeria tenella with added gentamicin and amphotericin B as preservatives (Novus, Inc., St. Louis, MO). The vaccine together with a high fibre diet (including rye and wheat middlings) was used to induce NE conditions within the gut. Control chicks were gavaged the same volume of a 0.9 % saline solution.
The basal diet for all groups was based on corn, soybean meal (SBM), wheat, rye, and wheat middling (Table 1). Feed samples were analysed for dry matter (AOAC 934.01, 2005), crude protein (AOAC 990.03, 2006), lipid (AOAC 920.39, 2003) and ash (AOAC 942.05, 2013).
Table 1. The ingredients and nutrients composition of control diet
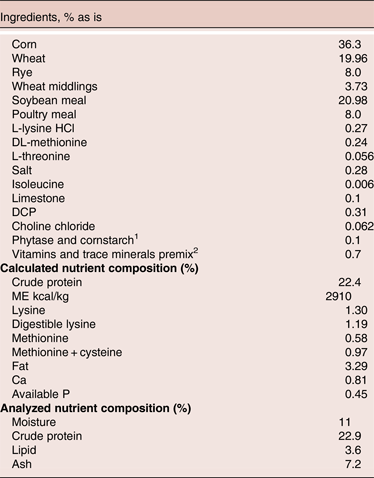
1includes phytase at 500 FTU/kg feed, test materials replaced corn starch, provides 2000 U xylanase/kg feed, 4000 U protease/kg, 200 U amylase/kg and 75000 cfu/g feed of Bacillus. Enzymes activities (xylanase and phytase) and DFM level were tested in the diets and all within the targeted levels
2 provide per kg feed: Iron: 371.72 mg; Manganese: 107.24 mg; Copper: 12.00; Selenium: 0.28 mg; Zinc: 85.08 mg; Iodine: 0.3 mg; Vit A: 8000.00IU; Vitamin D: 3721.44 IU; Vitamin E: 86.15 mg; Vitamin K: 2.41 mg; Biotin: 0.93 mg; Choline: 2000.00 mg, Folic acid: 10.19 mg; Niacin: 109.11 mg; Panto acid: 32.01 mg; Pyridoxine: 17.29 mg; Riboflavin: 26.23 mg; Thiamin: 27.30 mg; Vit B12: 0.03 mg
Observations, sampling and measurements
Birds (weighed on a pen basis) and feeders were weighed on days 0, 12 and 21. BWG and feed intake were measured and FCR was calculated. Mortality was recorded daily. Calorie conversion ratio was calculated as energy consumed (kcal) per kg BWG.
One bird per pen was bled and killed by cervical dislocation on days 12 and 21 for post-mortem analysis. Blood smears were made and plasma was isolated and stored frozen at −20°C prior to analysis. Plasma haptoglobin-like protein activity and alpha-1-acid glycoprotein concentrations were analyzed according to manufacturer instructions, using commercial Enzyme-linked immunosorbent assay (ELISA) kits (Phase Haptoglobin Kit #TP801, Tridelta Diagnostics, Morris Plains, NJ, USA; alpha 1 acid glycoprotein ELISA Kit (ab157690), Abcam, Cambridge MA). Blood differential counts were measured, according to the method published by Lucas and Jamroz (Reference Lucas and Jamroz1961).
Intestinal immunological analysis
Tissue samples from the midpoint of the duodenum, jejunum, ileum, and left caecum were dissected and stored in RNA storage solution (RNA Later, SigmaAldrich, St Louis, MO) at −80°C. Cytokine (Interleukin (IL)-1β, IL-6, IL-10 and Transforming growth factor (TGF)-β) levels were quantified by real time reverse transcriptase PCR (qPCR) as described previously by Meriwether et al. (Reference Meriwether, Humphrey, Peterson, Klasing and Koutsos2010). Briefly, total RNA was isolated from tissue samples using RNAgents Total RNA Isolation System (Promega, Madison, WI) according to manufacturer's instructions and reverse transcribed using Promega Reverse Transcription System (#A3500). Quantitative real-time PCR analysis was performed using a Roche Lightcycler (Roche Diagnostics #2 011 468, Mannheim, Germany). Cytokine data were expressed as 40 minus the CT value and were corrected for translation efficiency by using the house keeping reference gene GAPDH.
Histology
Additional tissue samples from the midpoint of the duodenum, jejunum, ileum, and left caecum were excised and placed in buffered formaldehyde and sent to an external laboratory (Idexx, West Sacramento CA) for fixing, sectioning, mounting and staining (haematoxylin and eosin). Slides were visualized using computer-aided light microscopy at magnifications between 10 and 100X using Image-Pro-Plus analysis software for the PC (Media Cybernetics, Del Mar, CA). The numbers of intraepithelial lymphocytes (IEL), thickness of the lamina propria; villous height (from the base of the lamina propria to the villous apex); villous width at its midpoint and crypt depth between adjacent villi were measured.
Data analysis
For performance data, each pen mean was designated an experimental unit. For blood and tissue analysis, the samples were pooled per pen and pen means were used for each experimental unit. All data were analysed as a 2 × 2 factorial design using the Fit Model platform of JMP 11; version 11 (SAS Institute Inc., Cary, NC, 2013). Tukey's HSD test was used for the comparison of treatment means. Significances were determined at 95% confidence limits (P < 0.05) and trends at 0.10 > P > 0.05.
Results
Performance parameters
The 2 × 2 factorial analysis showed that coccidial challenge did not affect feed intake (FI) but reduced bodyweight gain (BWG) (P < 0.05) and increased Feed conversion ratio (FCR) (P < 0.05) and calorie conversion ratio (P < 0.05) compared to the unchallenged birds. The addition of XAP + DFM did not have a significant impact on FI or BWG during starter and grower phases, however it reduced FCR (P < 0.05) and calorie conversion (P < 0.05) compared to control groups in all phases. Overall 1–21 data showed that XAP + DFM treatment improved BWG (34.1 vs 33 g/d, P < 0.05) and reduced FCR (1.35 vs 1.28, P < 0.05) compared to control (Table 2).
Table 2. Effect of dietary supplementation of XAP (xylanase, amylase and protease) and DFM (Bacillus spp.) combination on performance of broilers with and without coccidia challenge. 1
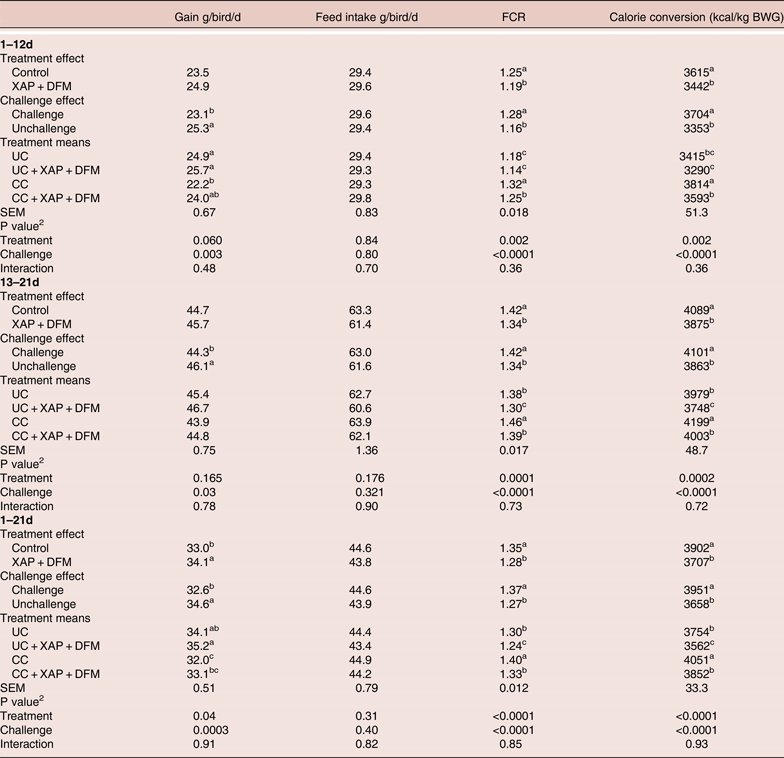
1 UC: unchallenged control; CC: coccidia challenged; UC + XAP + DFM: unchallenged control +enzymes and DFM; CC + XAP + DFM : challenged control + enzymes and DFM
2 P values determined by the general model
a,b, different superscripts in the same column indicate significant difference at P<0.05 based on treatment mean comparison
No XAP + DFM treatment x challenge interactions were found, however, XAP + DFM treatment improved performance to a larger extent under a challenged situation in the starter phase. During the 1–12days period, XAP + DFM treatment improved BWG by 3.4 and 8% and reduced FCR by 3.5 and 5.8%, in the absence and presence of challenge respectively. Calorie conversion ratio was significantly lower (P < 0.05) in CC + XAP + DFM treatment compared to CC in all phases, while CC + XAP + DFM reduced calorie conversion ratio in phase 2 feeding and overall period compared to UC. During 1–12 days, birds in XAP + DFM treatment used 125 and 221 kcal/kg less energy to produce one kg BWG, under unchallenged and challenged condition, respectively.
No mortality was observed in the study, however, at 21 day, less abnormality was observed in XAP + DFM treatment compared to the challenge control group (challenged control had 11 pasty cloaca, three severe tibia dischondroplasia and one with poor feathering; while XAP + DFM treatment had five pasty cloaca and one haematoma on wing).
Intestinal histological parameters
The histology results are summarised in Table 3. Based on 2 × 2 factorial analyses, the challenged group showed reduced (P < 0.05) villus height in the duodenum, increased duodenal, jejunal and caecal IEL counts, and increased jejunal and ileal lamina propria thickness at seven days post-infection. The XAP + DFM treatment showed numerically improved villi height in all digestive sections (between 6–11%) but was not significantly different (P > 0.05) than the control treatment. XAP + DFM treatment x challenge interaction was found for ileal IEL, where CC + XAP + DFM treatment reduced (P < 0.05) IEL compared to the CC and UC + XAP + DFM treatment tended to increase IEL compared to UC.
Table 3. Effect of dietary supplementation of XAP (xylanase, amylase and protease) and DFM (Bacillus spp.) combination and coccidia challenge on intestinal histology in broilers on day 12. 1
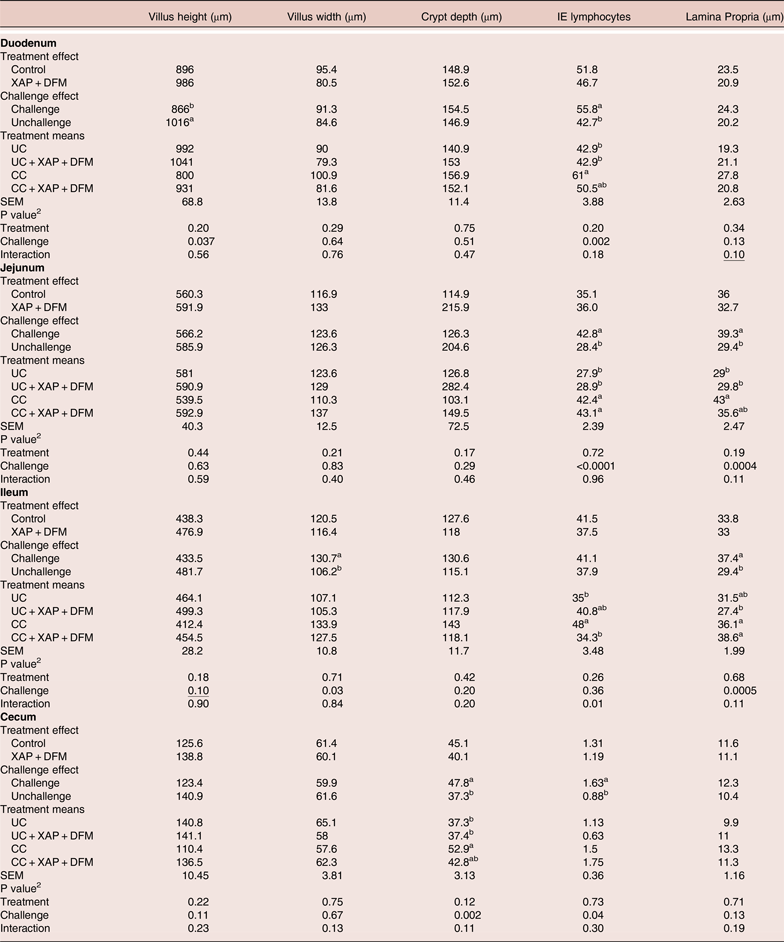
1 UC: unchallenged control; CC: coccidia challenged; UC + XAP + DFM : unchallenged control + enzymes and DFM; CC + XAP + DFM: challenged control + enzymes and DFM
2 P values determined by the general model a,b, different superscripts in the same column indicate significant difference at P < 0.05 based on treatment mean comparison
At 16 days post-infection (day 21), coccidia challenge reduced villi height (P < 0.05) in the duodenum, ileum and caecum and increased crypt depth in jejunum and ileum. This was associated with increased (P < 0.05) IEL in the duodenum, jejunum and ileum, and increased lamina propria thickness in the jejunum, ileum and caecum. XAP + DFM treatment reduced (P < 0.05) duodenal IEL. No XAP + DFM treatment x challenge interactions were observed (Table 4).
Table 4. Effect of dietary supplementation of XAP (xylanase, amylase and protease) and DFM (Bacillus spp.) combination and coccidia challenge on intestinal histology in broilers on day 21. 1
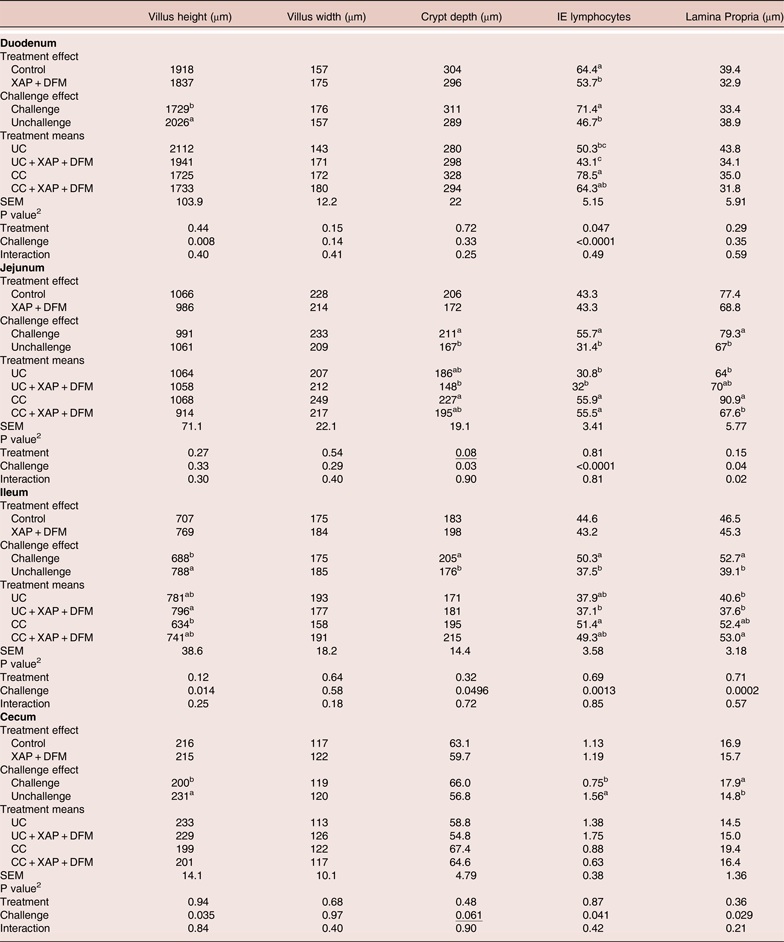
1 UC: unchallenged control; CC: coccidia challenged; UC + XAP + DFM : unchallenged control + enzymes and DFM; CC + XAP + DFM: challenged control + enzymes and DFM
2 P values determined by the general model
a,b, different superscripts in the same column indicate significant difference at P < 0.05 based on treatment mean comparison
Intestinal mucosal cytokines
At seven days post-infection (day 12), challenge increased (P < 0.05) levels of IL-6 mRNA in the duodenum and jejunum and IL-1β mRNA in the duodenum and reduced (P < 0.05) levels of duodenal IL-10 (Figure 1). The CC + XAP + DFM treatment group showed numerically (P > 0.05) reduced mRNA for IL-6 (−13.5%) and IL-1β (−7%) in the duodenum along with lower (−13%) jejuna TGF-β compared to CC birds.

Figure 1. Effect of dietary supplementation of XAP (xylanase, amylase and protease) and DFM (Bacillus spp.) combination on intestinal mucosal cytokine mRNA levels (corrected for GAPDH) with and without coccidia challenge at day 12. UC: unchallenged control; CC: coccidia challenged; UC + XAP + DFM: unchallenged control + XAP + DFM; CC + XAP + DFM: challenged control + XAP + DFM; C+ = mean effect of challenged treatment; C- = mean effect of unchallenged treatment. a,b, different superscripts in the same digestive section indicate significant difference at P < 0.05. Data from cecal samples are not included due to large number of undetectable values.
At 16 days post-infection (day 21), challenged birds showed reduced (P < 0.05) levels of TGF-β mRNA in duodenal and cecal sites; reduced IL-10 in ileum; increased mRNA for IL-6 (jejunum and ileum) and IL-1β at ileal and caecal sites. CC + XAP + DFM group showed reduced (P < 0.05) jejunal IL-6 mRNA and increased (P < 0.05) cecal TGF-β compared to the CC birds (Figure 2). Treatment x challenge interaction (P < 0.05) was found for ileal TGF-β, i.e. CC + XAP + DFM birds tended to decrease levels of ileal TGF-β mRNA, while in the presence of challenge CC + XAP + DFM birds tended to increase TGF-β levels.

Figure 2. Effect of dietary supplementation of XAP (xylanase, amylase and protease) and DFM (Bacillus spp.) combination on intestinal mucosal cytokinem RNA levels (corrected for GAPDH) with and without coccidia challenge at day 21. UC: unchallenged control; CC: coccidia challenged; UC + XAP + DFM: unchallenged control + XAP + DFM; CC + XAP + DFM: challenged control + XAP + DFM; C+ = mean effect of challenged treatment; C- = mean effect of unchallenged treatment. a,b, different superscripts in the same digestive section indicate significant difference at P < 0.05.
Plasma acute phase proteins (APP)
At seven days post-infection (12 day), challenged birds had increased α−1-acid glycoprotein and haemopexin (P < 0.05) levels in the plasma (Figure 3). XAP + DFM treatment reduced (P < 0.05) α−1-acid glycoprotein and tended to reduce (P = 0.066) haemopexin levels in the plasma. When comparing treatment means, the CC group showed significantly (P < 0.05) higher levels of APP, α−1-acid glycoprotein and haemopexin at seven days post-infection than the UC group. CC + XAP + DFM birds had lower levels of (P < 0.05) α−1-acid glycoprotein than the CC group. There was a tendency for a treatment x challenge interaction for α−1-acid glycoprotein at seven days post-infection (P = 0.055).

Figure 3. Effect of dietary supplementation of XAP (xylanase, amylase and protease) and DFM (Bacillus spp.) combination and coccidia challenge on plasma APP at day 12 and 21. UC: unchallenged control; CC: coccidia challenged; UC + XAP + DFM: unchallenged control + XAP + DFM; CC + XAP + DFM: challenged control + XAP + DFM; T- = mean effect of control treatment; T+ = mean effect of test treatment; C+ = mean effect of challenged treatment; C- = mean effect of unchallenged treatment. a,b, different superscripts in the same digestive section indicate significant difference at P < 0.05. A,B, different superscripts in the same digestive section indicate tendency at P < 0.10.
At 16 day post challenge (day 21), there was a tendency of increased α−1-acid glycoprotein (P = 0.053) and haemopexin (P = 0.055) levels in the plasma in the challenge groups compared to unchallenged groups (Figure 3).
Blood leukocytes
At both time points coccidia challenge increased (P < 0.05) heterophil counts and heterophil to lymphocyte (H/L) ratios while eosinophil counts were reduced. No significant differences (P > 0.05) were found in blood cell counts between control and test groups (Table 5).
Table 5. Effect of dietary supplementation of XAP (xylanase, amylase and protease) and DFM (Bacillus spp.) combination and coccidia challenge on blood leukocyte %. 1
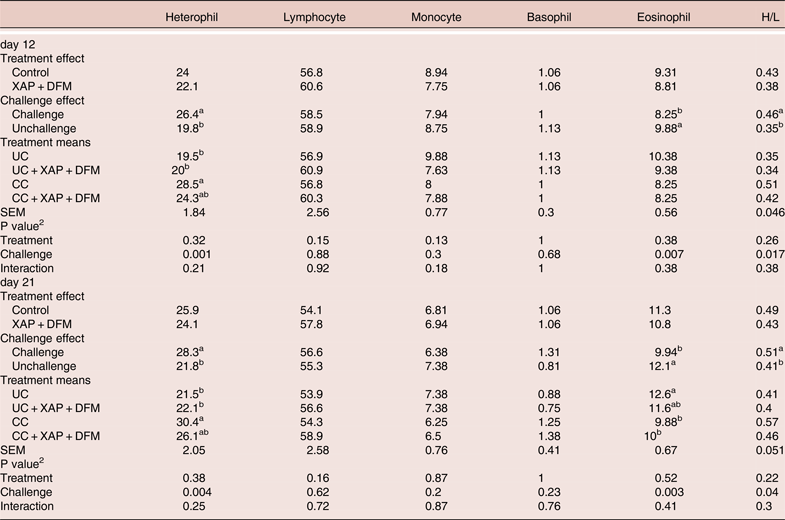
1 UC: unchallenged control; CC: coccidia challenged; UC + XAP + DFM: unchallenged control + enzymes and DFM; CC + XAP + DFM: challenged control + enzymes and DFM
2 P values determined by the general model
a,b, different superscripts in the same column indicate significant difference at P < 0.05 based on treatment mean comparison
Discussion and conclusions
In the current investigation, local intestinal responses to oocyst challenge led to reduced villi height, and elevated levels of pro-inflammatory markers IL-6 and IL-1β in various intestinal sites. The systemic response included increased plasma APP such as α−1-acid glycoprotein and haemopexin, along with blood heterophil counts. Both IL-6 and IL-1β are cytokines released from innate immune cells, including tissue macrophages and heterophils, in response to microbial associated molecular patterns (MAMPs). These cytokines possess many effector functions including the recruitment of more innate cells to the site of infection and stimulation of the APP response by hepatocytes. Simultaneous increases in APP levels and relevant cytokines were documented in this study in response to challenge. APP are proteins whose levels fluctuate in response to inflammatory responses; their production is stimulated by inflammatory cytokines and thus can be used as markers of inflammation (Adler et al., Reference Adler, Peng, Peng and Klasing2001). Haemopexin is responsible for scavenging and transporting free haeme to the liver to avoid both oxidative stress and prevent pathogens from utilising the iron for growth. Increased H/L ratio has been seen in increased stress conditions (Vleck et al, Reference Vleck, Vertalino, Vleck and Bucher2000) and can be induced by inflammatory cytokines (Klasing, Reference Klasing1991).
Challenge was associated with villi atrophy (Tables 4–6). The current results are in agreement with other studies. For example, coccidia challenge decreased weight gain, feed efficiency, increased duodenal lamina propria thickness and reduced villus height in chicks (Klasing et al., Reference Klasing, Adler, Remus and Calvert2002). Additionally, sub-clinical pathogenic challenge compromises intestinal integrity, induces acute phase responses and depresses growth performance (Klasing, Reference Klasing2007; Klasing and Johnstone, Reference Klasing and Johnstone1991). A number of studies have demonstrated the ability of Bacillus based DFM to dampen potentially detrimental immune responses (Lee et al., Reference Lee, Lillehoj and Siragusa2010a,b; Waititu et al., Reference Waititu, Yitbarek, Matini, Echeverry, Kiarie, Rodriguez-Lecompte and Nyachoti2014). Lee et al (Reference Lee, Lee, Lillehoj, Li, Jang, Babu, Park, Kim, Lillehoj, Neumann, Rehberger and Siragusa2010b) observed that supplementation with Bacillus spp. in the diet for broilers reduced serum α−1-acid glycoprotein, splenic and intestinal IEL numbers compared to the non-supplemented control groups.
Table 6. Statistics output related to effect of dietary treatments on intestinal mucosal cytokine mRNAs (Figure 1 and 2) and plasma acute phase protein (Figure 3)
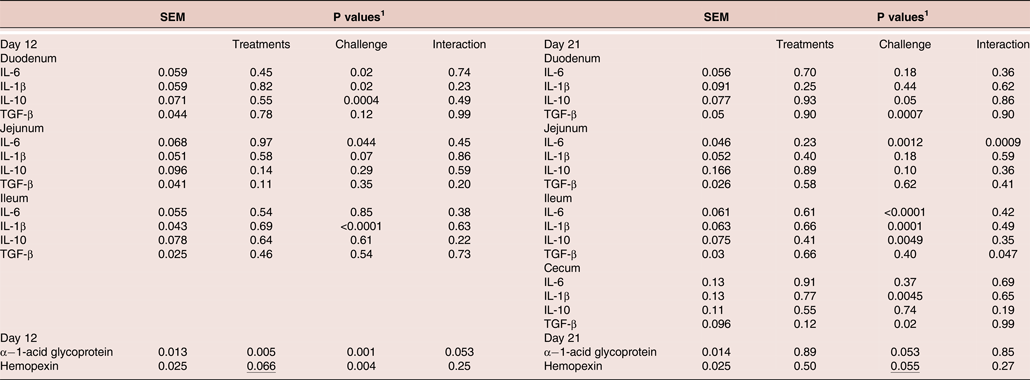
1 P values determined by the general model
Cell mediated immunity is likely to play a key role in the avian immune response to Eimeria spp. challenge (Lillehoj and Trout, Reference Lillehoj and Trout1996). This was supported in the current study which identified increased IEL at multiple intestinal sites following challenge. IEL reside at the intestinal basolateral epithelial surface and are thought to contribute to maintaining epithelial integrity (Inagaki-Ohara et al., Reference Inagaki-Ohara, Dewi, Hisaeda, Smith, Jimi, Miyahira, Abdel-Aleem, Horii and Nawa2006). In the current study, challenge also increased the lamina propria thickness in jejunum, ileum and caecum. The lamina propria is rich in macrophages and lymphoid cells that make it a key place for immune responses, increased thickness could be a possible sign of increased plasma and infiltration of effector cells. As expected, pro-inflammatory cytokine mRNA (IL-1β and IL-6) increased with challenge while anti-inflammatory cytokines (IL-10 and TGF-β) were reduced.
In this study, the XAP + DFM treatment was shown to limit the inflammatory response induced by coccidia challenge as indicated by significantly reduced APP levels, reduced IEL numbers and lamina propria thickness, and other inflammatory markers such as duodenal IL-6 at day 21 compared to challenged control. A few significant treatments x challenge interactions were found for IEL numbers, lamina propria thickness, pro- and anti-inflammatory cytokines and APP. There was a clear general trend of higher villus height, lower IEL numbers, lamina propria thickness and blood heterophil content in XAP + DFM treatment compared to challenged control during 1–12 days. It was observed that BWG (R2 = 0.96) or FCR (R2 = 0.98) was highly correlated to duodenum villus height during 0–12 days, when challenge was applied. Challenged group had lowest villus height, which was related to the highest FCR, while XAP + DFM treatment showed numerically higher villus height that corresponded to a significantly lower FCR compared to challenged control.
In the current study, the mild coccidia challenge did not result in reduced feed intake, however, weight again and feed efficiency were reduced. This could be partially due to the amount of water soluble NSP in the diet creating a highly viscous condition in the small intestine thus reduced nutrients digestion and feed efficiency, the effect might be more pronounced under challenged conditions. The reduced feed efficiency is perhaps also related to the intestinal histological changes and immune responses described above. These changes can consequently result in: 1) a reduction in the absorption area due to villi atrophy and thus reduce utilisation of nutrients, 2) increased energy and nutrient cost for the immune response, including the production of APP. Iseri and Klasing (Reference Iseri and Klasing2014) estimated the amount of nutrients needed by the systemic immune system using lysine as a metric. The immune system (leukocytes plus protective proteins) in a healthy chicken contains about 0.4% of the bodies lysine, which is equivalent to the amount of lysine in 5.4% of a pectoralis muscle. During the acute phase of a robust immune response the amount of lysine in the immune system doubles. The lysine needed for the hypertrophy of the liver, which facilitates the production of APP, together with that needed for leukocytes and protective proteins results in the diversion of lysine equals the amount found in about 20% of a pectoralis muscle. Increased energy use to support an immune response was observed in the current study, as shown in Table 2. During 1–12days at which time the response to coccidia challenge occurred, the energy used for maintenance clearly increased (chicks used 221 kcal more energy to produce one kg of body weight vs control). It has previously been suggested that sub-clinical pathogenic challenge increases the energy cost due to perpetual activation of acute phase immune responses (Applegate et al., Reference Applegate, Klose, Steiner, Ganner and Schatzmayr2010).
Supplementation with XAP + DFM combination reduced the negative impacts of a coccidia challenge by reducing levels of inflammatory response and maintaining performance comparable to unchallenged control. Although treatments did not have any impact on mortality, XAP + DFM treatment maintained better health status as indicated by reduced number of pasty cloaca and severe TD. Dietary supplementation with DFM has frequently been shown to improve intestinal integrity (Applegate et al, Reference Applegate, Klose, Steiner, Ganner and Schatzmayr2010). For example, it was observed that Bacillus subtilis increased ileal villi height in broilers (Lee et al., Reference Lee, Lee, Lillehoj, Li, Jang, Babu, Park, Kim, Lillehoj, Neumann, Rehberger and Siragusa2010b) and in an adult male layer strain (Samanya and Yamauchi, Reference Samanya and Yamauchi2002). In broilers challenged with coccidia or with low sanitation (built up litter) DFM increased intestinal epithelial integrity (Murugesan, Reference Murugesan2013). DFM can be effective in reducing pathogen colonisation including C. jejuni, E coli, Salmonella spp and E. acervulina (Dalloul et al., Reference Dalloul, Lillehoj, Shellem and Doerr2003; Willis and Reid, Reference Willis and Reid2008). Dalloul et al., (Reference Dalloul, Lillehoj, Shellem and Doerr2003) showed that with a coccidia infection challenge, DFM supplementation improved BWG, feed efficiency and reduced mortality in birds challenged with E. acervulina. However, not all studies have consistently shown improved growth performance with DFM supplementation. Differences between studies may be for a number of reasons including: host status, genetics, experimental design and microbiota. It has been suggested that reduced pathogenic load in relatively clean research environments might be one of the possible reasons for failure of a positive impact of DFM (Edens, Reference Edens2003, Murugesan, Reference Murugesan2013).
Nutritional management such as using XAP and DFM can improve intestinal integrity and increase energy efficiency under sub-clinical pathogenic challenge (Murugesan, Reference Murugesan2013). With increasing feed cost, use of more complex diets with increased inclusion of plant by-products has increased the need for enzymes in the diet. Recent studies have demonstrated a benefit of using a combination of XAP and DFM (Romero et al., Reference Romero, Indrakumar and Ravindran2013; Walsh et al., Reference Walsh, Romero, Indrakumar and Ravindran2013). Romero et al. (Reference Romero, Indrakumar and Ravindran2013) reported that this dietary treatment improved energy and nutrient digestibility in broilers at 21 days of age. The enzymes and DFM combination increased ileal digestible energy to a larger extent than when the supplemental products were used individually. It has been suggested that the enzymes and DFM may have complementary effects on nutrient digestibility in broilers. Similarly, Murugesan (Reference Murugesan2013) showed a significant interaction between XAP enzymes and DFM for apparent ileal digestibility of starch, crude protein, and amino acid, indicating that both additives increased the digestibility in a non-additive manner. An additive response was observed for AMEn when the enzymes were used in combination with DFM. FCR was reduced by supplementation of enzymes or DFM, however the effect of the combination was higher than the additives used individually. In first-cycle laying hens, it was reported that supplementation of the XAP enzymes and DFM (3 strains of Bacillus spp.) combination in corn-SBM-DDGS based diets increased energy digestibility, enhanced gut integrity, and reduced zoonotic pathogen load (Murugesan, Reference Murugesan2013). Supplementation of enzymes improved ileal nutrient uptake, while the combination of enzymes and DFM increased ileal mucin mRNA levels, colon trans-epithelial electrical resistance (TEER), and reduced endotoxin transport and Campylobacter spp. colonisation, indicating improved intestinal integrity. Improved feed efficiency (Bansal et al., Reference Bansal, Singh and Sachan2013) and growth performance (Müdüllü and Tuncer, Reference Müdüllü and Tuncer2001) by supplementing DFM and enzyme combinations have also been reported in literature studies. Momtazan et al. (Reference Momtazan, Moravej, Zaghari and Taheri2011) found that there was an interaction between an enzyme complex containing β-glucanase, α-amylase, cellulase, protease and lipase and a DFM for BWG, FCR and the relative weight of the duodenum. The authors concluded that the combination of the enzyme complex and DFM can improve the performance more than either supplement used on its own. One hypothesis is certain feed enzymes may act in a prebiotic fashion, providing suitable substrates to the probiotic strains and the favoured micro-organisms. Further work is needed to determine the products of enzyme activity and to assess the probiotic strains preference for these substrates to support growth.
The current study showed that the XAP and DFM combination improved energy efficiency under both challenged and unchallenged conditions. Although no significant interaction was found, the calorie conversion ratio (kcal/kg BWG) was 125 and 221 kcal lower in XAP and DFM combination treatment under non-challenge and coccidia challenge conditions, respectively, during 1–12d when challenge was induced. This implies that the combination may be more effective under challenge. A meta-analysis of eight trial studies (Dersjant-Li et al., Reference Dersjant-Li, Romero, Wealleans and Awati2014) demonstrated that the XAP enzymes in combination with 3 strains of Bacillus significantly improved feed efficiency and calorie conversion ratio (kcal/kg BWG). It was shown that the impact of the feed additives combination was related to the challenge conditions: the energy saving per kg body weight gain was estimated at 84 and 708 kcal/kg body weight gain under low and severe NE challenged situation respectively. In the current study a mild challenge was used which may explain the lack of interactions on calorie conversion. Additionally, diets containing viscous grains such as wheat, rye and wheat middling are known to predispose birds to NE and thus in the case of the current study may contribute to the challenge model. The improved energy efficiency observed in both non-challenge and challenged conditions may be partially explained by the increased enzyme substrate availability found in the high NSP containing diets.
The improved conversion of dietary energy to body weight by supplementing enzymes and DFM was associated with changes in intestinal morphology and immune response to a coccidial challenge. The results indicated that the supplementation of XAP and DFM reduced the severity of the coccidial challenge, mitigating the amount of intestinal inflammation and decreasing the energy requirement for the immune response. This is likely the reason for improved feed efficiency to levels comparable to the unchallenged control.
By 16 days post-challenge, prolonged immune responses were still observed in CC birds, including increased IEL numbers, IL-6 in jejunum and ileum, increased IL-10 in ileum and reduced TGF-β in duodenum. In the challenged birds, the XAP + DFM treatment reduced IE lymphocytes numbers in duodenum, reduced IL-6 in jejunum, indicating a prolonged reduction of the pro-inflammatory response. In the intestinal tract an inflammatory response could have detrimental effects on performance as tissue damage reduces function and energy is diverted away from bird growth. Interestingly, in the current study, an XAP + DFM treatment x challenge interaction for ileal TGF-β indicated that under non-challenge conditions, treatment tended to reduce the anti-inflammatory marker TGF-β, but during challenge the treatment increased TGF-β production which correlates with reduced pro-inflammatory markers. This may suggest that the combination of XAP and DFM resulted in an immunological balance appropriate for optimal energy availability for production.
In summary, the results implied that dietary xylanase, amylase, protease enzymes and Bacillus DFM combination treatments may reduce the impact of coccidia challenge, maintain the inflammatory/anti-inflammatory balance and reduce the extent of negative changes in the absorptive epithelium of the intestines. Lower levels of inflammation due to the combination of XAP and DFM were indicated by lower levels of the APP in blood plasma. This was correlated to improved growth and energy efficiency in broilers.
In conclusion, supplementation of XAP + DFM improved BWG and reduced FCR in broilers under both challenge and unchallenged conditions during 0–21 days. Under mild coccidia challenge, XAP + DFM treatment reduced inflammatory response and maintained better health status resulting a greater improvement in energy efficiency.
Acknowledgements
The authors acknowledge the care for the birds and assistance in conducting the experiments provided by the staff of the lab of Dr. Kirk Klasing, Department of Animal Science, University of California Davis.
Declaration of interest
Yueming Dersjant-Li, Kirsty Gibbs and Ajay Awati are employees of Danisco Animal Nutrition, DuPont Industrial Biosciences.










