Introduction
Consanguineous marriage (CM) or cousin marriage is defined as marriages performed between people descended from common ancestors with the same stock or close genealogical kin (Schwendinger-Schreck Reference Schwendinger-Schreck2013). CM can be classified by the level of relationship between spouses. First degree; among parents–offspring, second degree; brother-sister who share one-eighth of their genes, and third-degree; among uncle–niece or distant relations who share one-quarter of genes (Morrison Reference Morrison2011; Islam Reference Islam2018). However, more than a billion people living in various geographical areas over the world who favour CMs have about 8.5% of children belonging to them (Modell and Darr Reference Modell and Darr2002). Marriages between first cousin and uncle–niece are more prevalent, which make up 20%–30% of all marriages. Cousin marriage is pervasive in Sub-Saharan African, Middle Eastern, South Asian, and Gulf countries (Bittles and Black Reference Bittles and Black2010). Further, Muslim-majority countries like Afghanistan, Lebanon, Egypt, and Saudi Arabia have a greater prevalence of CMs as this is a common practice in the Muslim religion (Tadmouri et al. Reference Tadmouri, Nair, Obeid, Al Ali, Al Khaja and Hamamy2009; Saify and Saadat Reference Saify and Saadat2012). Apart from the Muslim religion, consistency exists in some Hindu societies, majority state of Southern India, 20%–45% of marriages take place between close relatives, and most often between uncles and nieces (Bittles Reference Bittles1994).
However, the distribution of CM varies with individual characteristics, e.g. socioeconomic status, educational qualification, and social factors such as geographical regions, religious beliefs, and cultural practices (Krishnamoorthy and Audinarayana Reference Krishnamoorthy and Audinarayana2001; Bittles Reference Bittles2002). A study from India revealed that 10% of CMs happened in India; South India contributed almost a quarter of all marriages in 2015–16 (Sharma et al. Reference Sharma, Kalam, Ghosh and Roy2021). The population of Dravidian South Indian states such as Andhra Pradesh, Karnataka, Kerala, and Tamil Nadu strongly favoured CM, established 2,000 years ago (Nilakanta Sastri Reference Nilakanta Sastri2005). Albeit in India, it is a common practice among Muslims, a higher proportion is also found among the Hindu population, especially among tribal and scheduled tribes (STs) (Rao and Murty Reference Rao and Murty1984). It has been reported that in Bengaluru and Mysore, which are two major cities in South Indian state of Karnataka, there were 21% Hindu marriages between uncle and niece (Bittles et al. Reference Bittles, Shami, Rao, Roberts and Bittles1992). Even now, in the mid-twenty-first century, one-third of tribal women and 25% of women aged 15–49 years in South India reported having CM (Sahoo et al. Reference Sahoo, Debnath, Mandal, Nagarajan and Appunni2021). However, the main reason for the popularity of cousin marriages being advanced in South India is due to the benefit of social stability, which in turn involves the maintenance of family property (Dronamraju Reference Dronamraju1964; Reid Reference Reid1973). Moreover, the consanguineous union tradition of a specific group and advantages related to dowry and close relations between couples, which lead to durable and stable marriage life, are the dominant reasons for such practices (Shenk et al. Reference Shenk, Towner, Voss and Alam2016).
Worldwide, more than 130 million infants are born each year and a considerable number of 13.5 million of these children have inbred parents (Teeuw et al. Reference Teeuw, Henneman, Bochdanovits, Heutink, Kuik, Cornel and Kate2010). Autosomal recessive disturbances and birth defects among offspring are the common results of CM (Fareed and Afzal Reference Fareed and Afzal2017; Anwar et al. Reference Anwar, Mourosi, Arafat and Hosen2020; Sharma et al. Reference Sharma, Kalam, Ghosh and Roy2021). Also, CM adversely affects fetal survival indicators and gives birth to health-conscious offspring (Afzal et al. Reference Afzal, Lund and Skovby2018). Previous studies have been reported that parents with high blood pressure likely to be at increased risk of hypertension (Ziada et al. Reference Ziada, Al Kharusi and Hassan2001; Bittles and Black Reference Bittles and Black2010). Congenital heart disease (CHD), which is thought to be a genetic factor in cousin marriage, can contribute to disease risk, especially in some parts of the world (Modell and Darr Reference Modell and Darr2002; Bittles Reference Bittles2008). A case-control study conducted in Pakistan found that consanguinity was responsible for about half of the CHD (Haq et al. Reference Haq, Jalil, Hashmi, Jumani, Imdad, Jabeen, Hashmi, Irfan, Imran and Atiq2011). Moreover, a family history of diabetes is genetically transferred to their offspring (Bener et al. Reference Bener, Zirie and Al-Rikabi2005, Reference Bener, Hussain and Teebi2007; Ramkumar et al. Reference Ramkumar, Sagayaraj and Sharma2018). A clinical study proved that consanguinity could build a congenital structure for multistage carcinogenesis by developing a homogeneous situation for a recessive tumour gene, which raises the risk for cancer (Bittles Reference Bittles2001).
India is now on track to experience a huge burden of non-communicable diseases (NCDs) such as hypertension, diabetes, heart diseases, cancer, and psychiatric disorders as the prevalence escalates rapidly (Arokiasamy Reference Arokiasamy2018). The NCDs contributed to around 60% of all the factors responsible for deaths in India in 2014 (World Health Organization 2005). A study estimated that India would lose $4.58 trillion as a result of the burden of NCDs and mental health issues between 2012 and 2030, over twice the Indian gross domestic product (Saxena et al. Reference Saxena, Bloom, Cafiero-Fonseca, Candeias, Adashi, Bloom, Gurfein, Jané-Llopis, Lubet, Mitgang and Carroll O’Brien2014). Thus, the growing burden of NCDs is a rising concern for policymakers. A shred of evidence was established on the negative effects of CMs on the health condition of their offspring. However, limited evidence exists on this issue at the national level. Using nationally representative data, the present study aimed to investigate the association between parent’s consanguinity and chronic illness among their children and grandchildren in India.
Data and methods
The study utilised the Longitudinal Aging Study in India (LASI), 2017–2018, Wave 1 data, coordinated by the Harvard T.H. Chan School of Public Health, the International Institute for Population Sciences, Mumbai and the University of Southern California. The survey collected extensive information on individuals’ physical, social, and cognitive health (72,250) aged 45 and above across all states and union territories of India (excluding Sikkim). The survey used a multistage stratified area probability cluster sampling design wherein a three-stage sampling design was used for rural areas and a four-stage sampling design for urban areas (International Institute for Population Sciences (IIPS) et al. 2020). The present study considered all 72,250 samples for the analysis. The unit of analysis is the children/grandchildren in the study, whose chronic illnesses are observed.
Variable description
The CM, which was the variable of interest in the study, was measured by the question “Is your current or former spouse related to you by blood (like a cousin)?”. Whoever responded yes was coded as 1 else 0. The adverse health conditions among children/grandchildren were considered as outcomes, which include psychotic disorder, hypertension, diabetes, heart disease, stroke, cancer, and also a history of birth defects or congenital disorders (among children only due to data unavailability).
The age of the respondents was taken as a continuous variable, including ages 45 and above, along with the spouses of respondents irrespective of age. The gender of the respondents was categorised as male and female. Education was measured by years of schooling as a continuous variable. The religion of the respondents was categorised as Hindu and other. The social class/caste of the respondents was recorded as the scheduled caste (SC)/ST, and others. The economic condition was measured by the monthly per capita expenditure (MPCE) quintile that had been constructed using household consumption data. It uses sets of 11 and 29 questions on the expenditures on food and non-food items, respectively. Food expenditure was collected based on a reference period of seven days, and non-food expenditure was collected based on reference periods of 30 days and 365 days. The MPCE was then classified as poorest, poorer, middle, richer, and richest.
Statistical analysis
The study variables were first summarised using descriptive statistics, which were mean, standard deviations (continuous variables), frequency distribution, and percentages (categorical variables). Bivariate analysis was carried out to examine the significant association between the covariates and the dependent variable CM. Independent t-tests were used for continuous variables, and chi-square tests for categorical variables.
Propensity score matching technique was used for estimating the relation between CM and the adverse health outcome among their children/grandchildren, by constructing similar observed characteristics in a control group to the treatment group. The conditional independence assumption (CIA) is one of the key assumptions of propensity score matching (PSM), which assumes that there are no confounding variables between the treatment and control groups, conditioned on observables. Under the CIA, the differences in adverse health outcomes among children/grandchildren between the two groups can be attributed to the consanguinity of parents. Being in a CM was the treatment effect and the adverse health outcomes on children/grandchildren were the outcomes.
Let Y 1i and Y 0i represent the adverse health outcomes among children/grandchildren of individual i in a CM and those who are not in it, respectively. The average treatment effect on the treated (ATT) is given by
where CM is the consanguineous marriage. However, the adverse health outcomes among children/grandchildren if their parents are not in a CM are not observed. One strategy is to assume that the CIA on these unobserved cases is conditioned on the observables for taking these missing counterfactual outcomes and is represented by
The propensity score p(X i ) is the probability that the individual is in a CM or not, given the background characteristics, which is used for the process of matching (Rosenbaum and Rubin Reference Rosenbaum and Rubin1983). Therefore, ATT can be rewritten as
In the PSM technique, another assumption is of the common support that ensures the probability of being treated and untreated for individuals with similar characteristics.
In the present study, a probit model was estimated at first taking CM as the dependent variable and the other socio-demographic characteristics as the covariates. The propensity score estimates p(X i ) were then used to construct the control groups. The nearest neighbourhood matching technique was used for selecting individuals from the control group that had propensity scores near to the individuals in the treatment group. Consider NT and NC as the number of observations in the treatment and control group units matched, respectively, then the estimated ATT is (Becker and Ichino Reference Becker and Ichino2002) given by
 $${\rm{ATT}} = {1 \over {{N_T}}}\sum\limits_{\left\{ {i:{\rm{C}}{{\rm{M}}_i} = 1} \right\}} {\left[ {{Y_i} - \sum\limits_{\left\{ {j:{\rm{C}}{{\rm{M}}_i} = 0} \right\}} {{w_{ij}}} {Y_j}} \right]} $$
$${\rm{ATT}} = {1 \over {{N_T}}}\sum\limits_{\left\{ {i:{\rm{C}}{{\rm{M}}_i} = 1} \right\}} {\left[ {{Y_i} - \sum\limits_{\left\{ {j:{\rm{C}}{{\rm{M}}_i} = 0} \right\}} {{w_{ij}}} {Y_j}} \right]} $$
where,
Ten nearest neighbour untreated individuals were selected that had a propensity score within the range of 0.01 of each of the treated individuals.
Results
Table 1 shows the descriptive statistics of the study variables. The mean age of the sample was around 60 years and the age range was between 18 and 116. The sample constituted of 58% females and 42% male. Majority of the samples lived in rural areas, that was around 68.2%, and only 31.8% of the sample lived in urban areas. Approximately 28% of the sample belonged to SCs or STs, and most samples belonged to the Hindu religion (82%). The average years of schooling of the sample was 4 years, with a range from 0 to 26 years. According to the distribution of the economic condition, the majority of the samples were distributed in the poorest to the middle quintiles. Around 24% of the samples reside in the southern region of India and 11.5% of the marriages were consanguineous. The sample had 3.3% of individuals with a history of birth defects or congenital disorders.
Table 1. Descriptive statistics of the study variables (N = 72,250)

a Grandchildren not included due to data unavailability.
The bivariate tests of association of the covariates with CM are presented in Table 2. Consanguinity was more prevalent among rural areas (71.05%) than urban (28.95%). It was also more prevalent in the southern region (43.46%) of India and among the Hindu religion (72.53%). The average years of schooling were found to be 4.3 years for individuals with CM. Apart from the richest quintile of MPCE, the poorest to middle quintile had 22% and the richer quintile had 22% of CMs.
Table 2. Bivariate tests of the covariates with consanguineous marriage

From Figure 1 we observed that, the diseases among the children and grandchildren of the individuals in consanguineous marriage were- 18.5% with heart diseases, around 14% with psychotic disorders and stroke; and 13.3% with diabetes, had consanguineous parents. Around 4.75% having a history of birth defects or congenital disorders had blood-related spouses. These diseases were also significantly associated with CMs.

Figure 1. Percentage of diseases among children and grandchildren having consanguineous parents; *** P < 0.01, **P < 0.05, *P < 0.10.
The spatial distribution of consanguinity marriage was found localised to South India (Figure 2a.). The highest prevalence was recorded in the state of Andhra Pradesh (28%) and the lowest in Kerala (5%) among South Indian states. A significant proportion of consanguinity was observed in the states of Arunachala Pradesh (22%), Gujarat (20%), and Jammu & Kashmir (16%). While consanguinity had been sporadic across other regions and states of India. In understanding the CM among different region groups (Figure 2b), it was found that in south India, consanguinity was more prevalent among Hindus. On the contrary, in the Muslim-majority states of Jammu and Kashmir and Lakshadweep, the prevalence was higher. Similarly in the eastern states of India, West Bengal, Bihar, and Jharkhand, consanguinity was more among the Muslim population. In the Northeastern states, consanguinity was more common among other religious groups. An interesting pattern can be observed in the states of Rajasthan, Gujarat, and Maharashtra, where consanguinity was significantly high among Hindus.

Figure 2. Distribution of consanguineous marriage in India, Longitudinal Aging Study in India (2017–2018). (a) prevalence by state; (b) prevalence by religious groups.
Propensity score estimation & covariate balancing test
Prior to the estimation of the effect of CMs on the diseases or disorders among their children and grandchildren, the results of the probit regression model are presented in Table 3. Almost all the estimated coefficients of the regression model were statistically significant, except for the poorer quintile in the MPCE covariate. The social economic and demographic variables were important covariates for determining consanguinity. The practice of CM in rural areas was 9.6% more likely than in urban areas. The SCs/STs were 20% less likely to practice consanguinity in comparison to other castes. The richer and richest MPCE quintiles were 7% and 4.3%, respectively, more likely to go for a CM than the poorest quintile. The individuals in the southern region (β: 0.524), belonging to Hindu religion (β: 0.182), had emerged to be strong predictors of CM.
Table 3. Multivariate Probit regression estimates taking consanguineous marriage as outcome variable
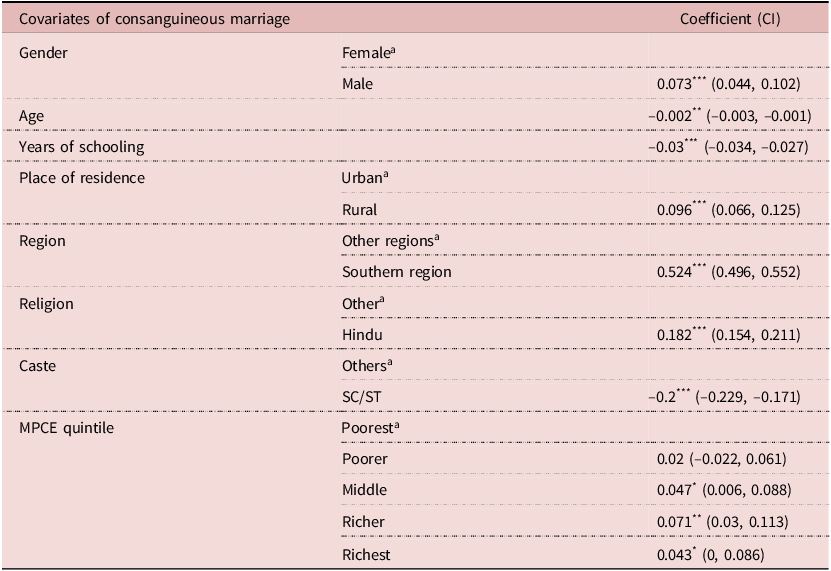
a Reference Category.
* P<0.10,
** P<0.05,
*** P<0.01.
In Table 4, the result of the covariate balancing test by the neighbourhood matching method is presented. Panel A shows the balancing property of the explanatory variables. It showed the mean difference between the individuals who practiced CM and those who did not and correspondingly the t-statistic value was given, which indicates the significant mean difference between the samples, and also the percentage reduction in bias was given. Overall, the tests were suggestive of the fact that the treated and the untreated groups were well-matched, and there was a significant reduction of bias for almost all the explanatory variables.
Table 4. Covariate balancing test using neighbourhood matching technique

*** P<0.01.
Panel B shows the overall test of balancing properties between the treatment and non-treatment groups. The Pseudo R-squared values from the probit regression model decreased from 0.043 in the unmatched sample to 0.001 in the matched sample, which suggests that there were no systematic differences existing between the individuals who were in a CM and those who were not, since the covariates had failed to explain variability in consanguinity. After matching, there was a considerable decrease in mean bias from 10.8 to 2.1, though the matching may not be the most perfect one as the likelihood ratio test was significant.
Main results after PSM
Consanguineous or blood-related marriages can lead to diseases and disorders among offspring. The study found that for individuals who were in CMs, there was 0.85% chance of their children and grandchildren developing psychotic disorders (Table 5). Similar results for heart disease as well, where their offspring will be 0.84% likely to develop heart disease. Interestingly, it was found that individuals in CMs have a 1.57% chance of developing hypertension. Additionally, there was 0.43%, 0.34%, and 0.14% likeliness of the children and grandchildren of the individuals having blood-related spouses to develop stroke, cancer, and diabetes, respectively. Around 4.55% of the individuals were having a history of birth defects or congenital disorders. The effect of consanguinity was significantly stronger in cases of developing psychotic disorders, birth defects, or congenital disorders and heart disease.
Table 5. Estimated average treatment effect on the treated of consanguinity on diseases or disorders among children or grandchildren for respondents who have blood-related spouses compared to those who do not have

a Grandchildren not included due to data unavailability.
* P<0.10,
** P<0.05,
*** P<0.01.
Discussion
Many people still practice CM, particularly in Asia, Africa, and South America (A. H. Bittles Reference Bittles1990, Reference Bittles1994). First cousin marriage is common in most Middle East, West and South Asia, including India (Hussain and Bittles Reference Hussain and Bittles1998; Bener and Hussain Reference Bener and Hussain2006; Sharma et al. Reference Sharma, Kalam, Ghosh and Roy2021). The purpose of this paper is to draw attention to the health implications and socio-demographic factors that control CM in India. We used the PSM technique to create well-balanced treatment and control groups based on various socio-demographic and probable disease variables as health outcomes (Bener and Hussain Reference Bener and Hussain2006).
In India, CM is predominant in religious believes (Oniya et al. Reference Oniya, Neves, Ahmed and Konje2019). Another key predictor of CM is regional culture wherein South India has 43%. In the same line, previous research has also found the highest prevalence of CM in the Southern states of India, where the Dravidian Hindus have been contracting close kin partnerships for over 2,000 years (Centerwall and Centerwall Reference Centerwall and Centerwall1966). Other studies suggest, in South India consanguinity is thought to offer major advantages in terms of compatibility between the bride and her husband’s family, which in turn restricts low or no dowry payments and thereby the family property remains undivided (Bittles et al. Reference Bittles, Mason, Greene and Rao1991; Padmadas and Nair Reference Padmadas and Nair2002; Banerjee and Roy Reference Banerjee and Roy2002; Ramkumar et al. Reference Ramkumar, Sagayaraj and Sharma2018). The result from our study shows a significant proportion of CM among Muslims. There are religious contexts, that can be exemplied from facts such as, the Prophet’s six wives, two of whom were biological relatives. He also married Ali, his paternal first cousin, to his daughter Fatima (Armstrong Reference Armstrong1991; Shieh et al. Reference Shieh, Bittles and Hudgins2012).
Despite India’s rising urbanisation and modernisation (Bhagat et al. Reference Bhagat, Roy and Sahoo2021), the current study found that CM was still prevalent. Although the predominance of CM is uncommon in urban areas, it is interestingly high among the higher social classes (non-SC/ST). However, a study by Sharma et al. Reference Sharma, Kalam, Ghosh and Roy2021 has found that non-SC/ST groups are 0.8 times less likely to get married to their first cousins (Sharma et al. Reference Sharma, Kalam, Ghosh and Roy2021). According to the present study, CM decreases as the number of years of education increases. This could be attributed to India’s urbanisation, which has resulted in increased educational attainment, family nuclearization, changes in occupational types, and possibly a greater ability to choose one’s life partner (Bhagat et al. Reference Bhagat, Roy and Sahoo2021). Similar findings from state of Tamil Nadu, have shown an inverse relationship between education and consanguineous union (Rao and Inbaraj Reference Rao and Inbaraj1977). Perhaps the Indian Government’s education programme, education to all, will impact India’s socio-demographic structure, and women will be able to choose their marital partners. The economic strata has been equated with the MPCE quintile, which suggests that people with a higher standard of living may have a better education level and more freedom to select marital partners and be aware of the negative effects of consanguinity (Mammen and Paxson Reference Mammen and Paxson2000). The present study has found the richest MPCE quintiles are more likely to marry consanguineously. This is corroborated by a few studies in which CM has been a feature of culture to limit the transfer of household wealth (Caldwell et al. Reference Caldwell, Reddy and Caldwell1983; Bittles et al. Reference Bittles, Shami, Rao, Roberts and Bittles1992; Mobarak et al. Reference Mobarak, Kuhn and Peters2013).
After adjusting for socio-demographic characteristics, the current study identified particular illnesses among the children and grandchildren of a consanguineous union. Various mental and congenital disorders were reported as statistically significant (P < 0.01) and have also been reported in other studies (al-Gazali et al. Reference al-Gazali, Dawodu, Sabarinathan and Varghese1995; Bittles Reference Bittles2003; Shaw Reference Shaw2018). Early life factors such as parental deprivation and low birth weight are well-recognised to be linked to poor mental health outcomes and consanguinity (Mumtaz et al. Reference Mumtaz, Tamim, Kanaan, Khawaja, Khogali, Wakim and Yunis2007; Lopez-Castroman Reference Lopez-Castroman2014). Further, children of consanguineous parents are also subjected to some stigma, particularly in areas where consanguinity is not the norm, and this stigma may harm their mental health (Bennett et al. Reference Bennett, Motulsky, Bittles, Hudgins, Uhrich, Doyle, Silvey, Scott, Cheng, McGillivray, Steiner and Olson2002; Maguire et al. Reference Maguire, Tseliou and O’Reilly2018). The history of birth defects has been observed among Pakistani immigrants in Norway (Stoltenberg et al. Reference Stoltenberg, Magnus, Lie, Daltveit and Irgens1997). Another major illness of CHD has been observed with consanguinity. The present study is also in line with the same result by Ramegowda & Ramachandra Reference Ramegowda and Ramachandra2006, where first-cousin and uncle–niece marriages were statically significant in increasing CHDs among their offspring (Ramegowda and Ramachandra Reference Ramegowda and Ramachandra2006). A study based on Kashmir found a high frequency of CHD among children with Down syndrome from a population with consanguineous union (Ashraf et al. Reference Ashraf, Malla, Chowdhary, Malla, Akhter, Rahman and Javed2010). Similarly, in a Muslim-majority country, Bangladesh, it has been observed that children of consanguinity union have more chances of congenital abnormalities (Anwar et al. Reference Anwar, Mourosi, Arafat and Hosen2020). These studies suggest that cardiac malformations are common among children and therefore discourage the association of consanguinity. Furthermore, diabetes has also been reported among children in India under consanguinity (Aravinda Reference Aravinda2019, p. 20). A study conducted by Bener et al, Reference Bener, Zirie and Al-Rikabi2005 reported higher odds of developing type-2 diabetes among CMs in Qatar (Bener et al. Reference Bener, Zirie and Al-Rikabi2005). Another study reported that 5.5% of the mothers (in consanguineous union) had affected offspring with diabetic mellitus (Bener and Hussain Reference Bener and Hussain2006). Additionally, in the present study, cancer and hypertension were also statistically significant (P < 0.10). Therefore, this study, with the support of previous literature, suggests that parental consanguinity is a risk factor for a variety of multifactorial issues, including cardiovascular disease, mental retardation, birth defects, diabetes, and various cancers, all of which can have an impact on reproductive outcomes (Oniya et al. Reference Oniya, Neves, Ahmed and Konje2019).
Conclusion
The risk of CM is high. Unfortunately, the general population appears to be unaware of the cons of CMs and possible links to the prevalence of associated illnesses. In India, the difficulty in communicating genetic risk information is that general-public pre-existing understandings of biological heredity and/or disease aetiology are ignorant and superficial, wherein bio-physical and psychological diseases are perceived as having environmental causes-accidents, infections, tragic life, God’s will, or the participation of hostile spirits. To address the risk of complicated illnesses underlying cultural features of close kin unions in India, adequate knowledge of Mendelian genetics is required, and more studies should be conducted to emphasise the importance of community education and medical, genetic, and social counselling services in enabling couples to make informed decisions about eligible spouses and reproductive options.
Data availability statement
The study uses secondary data which is available on reasonable request through https://www.iipsindia.ac.in/content/lasi-wave-i.
Acknowledgements
The authors cordially acknowledge IIPS for providing us with the LASI Wave 1 dataset for conducting the study.
Author contribution
SK: Conceptualisation, methodology, validation, formal analysis, investigation, and writing (original draft preparation). AJ: Conceptualisation, methodology, validation, formal analysis, investigation, and writing (original draft preparation). All authors have contributed equally in each section of the study, and read and approved the final manuscript.
Funding statement
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Competing interests
The authors declare no conflict of interest among them.
Ethical standard
The analysis is based on secondary data available in the public domain for research; thus, no approval was required from any institutional ethical review board. The survey agencies conducted the fieldwork with prior consent from the respondents. The LASI was conducted in accordance with the relevant ethical guidelines and regulations.









