Introduction
The relative lengths of the 2nd and 4th digits (digit ratio; 2D:4D) is thought to be a negative correlate of prenatal testosterone (T) and a positive correlate of prenatal oestrogen (E) (Manning et al., Reference Manning, Scutt, Wilson and Lewis-Jones1998; Manning, Reference Manning2002). The evidence for this, in humans (Breedlove, Reference Breedlove2010; Manning et al., Reference Manning, Kilduff and Trivers2013; Sadr et al., Reference Sadr, Khorashad, Talaei, Fazeli and Honekopp2020) and non-human animals (Zheng & Cohn, Reference Zheng and Cohn2011; Auger et al., Reference Auger, Le Denmat, Berges, Doridot, Salmon, Canivenc-Lavier and Eustache2013), has been the subject of debate over the last 20 years (see Swift-Gallant et al., Reference Swift-Gallant, Johnson, Di Rita and Breedlove2020, in support, and McCormick & Carré, Reference McCormick and Carré2020, in dispute). Presently, there is a substantial and growing body of evidence that links 2D:4D and prenatal sex steroids (Manning & Fink, Reference Manning, Fink, Shackelford and Weekes-Shackelford2018a,b). However, the source of the sex steroids, i.e. whether from the mother and/or the fetus, remains unclear. Maternal influence on fetal 2D:4D may be inferred from factors that influence maternal fitness.
The present report considers the effect of inequality of parental income on the 2D:4D of their children. It focuses on evolutionary influences on both the mother and her children by considering the Trivers-Willard hypothesis (Trivers & Willard, Reference Trivers and Willard1973). This hypothesis rests on the assumption that sons from high-resource mothers have higher reproductive success than daughters from high-resource mothers, while daughters from low-resource mothers will be more reproductively successful than sons from low-resource mothers. In the present study, the proxy for the maternal resource is parental income such that parents with high income are expected to invest in sons while those with low income will invest in daughters. Considering this through the lens of the Trivers-Willard hypothesis, it is suggested that if maternal sex steroid levels cannot be adjusted to the sex of the fetus then mothers with high income will masculinize their children in utero while mothers with low income will feminize their children.
Digit ratio is sexually dimorphic (males < females; Manning et al., Reference Manning, Scutt, Wilson and Lewis-Jones1998; Manning, Reference Manning2002). Adult-typical ratios of the lengths of 2D and 4D are attained very early in fetal development (i.e. before the end of the 1st trimester; Garn et al., Reference Garn, Burdi, Babler and Stinson1975), and the sex difference in 2D:4D is established at this stage (Malas et al., Reference Malas, Dogan, Evcil and Desdicioglu2006; Galis et al., Reference Galis, Ten Broek, Van Dongen and Wijnaendts2010). In humans, direct evidence for a causal relationship between 2D:4D and T:E levels at the end of the first trimester is difficult to obtain. However, sex differences in digit ratios are common among the tetrapods and may have arisen as early as the transition from aquatic to terrestrial locomotion, i.e. in the common ancestor(s) to reptiles, birds and mammals (Manning, Reference Manning2002; Lofeu et al., Reference Lofeu, Brandt and Kohlsdorf2017). Exogenous T and/or E have been shown to masculinize or feminize 2D:4D in non-human animals, including the larval stages of Anurans (Lofeu et al., Reference Lofeu, Brandt and Kohlsdorf2017), rodents (Talarovicova et al., Reference Talarovicova, Krskova and Blazekova2009; Auger et al., Reference Auger, Le Denmat, Berges, Doridot, Salmon, Canivenc-Lavier and Eustache2013; Lofeu et al., Reference Lofeu, Brandt and Kohlsdorf2017) and primates (Abbott et al., Reference Abbott, Colman, Tiefenthaler, Dumesic and Abbott2012). A detailed consideration of the effects of sex steroids (and sex steroid blockers) on the fetus of the mouse has shown that exogenous T masculinizes 2D:4D and E feminizes 2D:4D, with these effects being restricted to a narrow developmental window (Zheng & Cohn, Reference Zheng and Cohn2011). In humans, fetal T concentrations and T:E ratios obtained by routine amniocentesis in the 2nd trimester have shown negative relationships with children’s 2D:4D (Lutchmaya et al., Reference Lutchmaya, Baron-Cohen, Raggatt, Knickmeyer and Manning2004; Ventura et al., Reference Ventura, Gomes, Pita, Neto and Taylor2013). High (feminized) 2D:4D has been reported for traits associated with low prenatal T, i.e. Klinefelter’s Syndrome (Manning et al., Reference Manning, Kilduff and Trivers2013; Chang et al., Reference Chang, Skakkebæk, Trolle, Bojesen, Hertz and Cohen2015), or low sensitivity to T (i.e. androgen insensitivity, Berenbaum et al., Reference Berenbaum, Bryk, Nowak, Quigley and Moffat2009). Low (masculinized) 2D:4D has been found in children with high prenatal T, i.e. congenital adrenal hyperplasia (CAH) (see for a review Sadr et al., Reference Sadr, Khorashad, Talaei, Fazeli and Honekopp2020).
Perturbations in prenatal sex steroids may be of fetal origin, e.g. in Klinefelter’s Syndrome and CAH. However, maternal sex steroids may also cross the placenta and influence fetal 2D:4D (Barona et al., Reference Barona, Kothari, Skuse and Micali2015; Manning & Fink, Reference Manning and Fink2017; Ellis et al., Reference Ellis, Eisenmann and Hoskin2018). For example, in Titi monkeys (Plecturocebus cupreus) right-hand 2D:4D is sexually dimorphic in a human-like pattern (males < females), and high maternal T and high T:E ratio are negatively related to 2D:4D (Baxter et al., Reference Baxter, Wood, Witczak, Bales and Higley2020). In humans, there are reports that traits correlated with maternal sex steroids are also related to children’s 2D:4D. Mothers with high waist-to-hip ratios (WHR; a trait that correlates with high T:E ratio) have children with masculinized 2D:4D (Manning et al., Reference Manning, Trivers, Singh and Thornhill1999; Manning, Reference Manning2002). Maternal T levels may be elevated also by exposure to sunlight with consequences for the 2D:4D of the children. Thus, low 2D:4D has been reported in children whose mothers experienced long day lengths during the 1st trimester of their pregnancies (Szwed et al., Reference Szwed, Kosinska and Manning2017). Maternal T levels assayed in the 2nd trimester were reported to be negatively related to their children’s 2D:4D (Ventura et al., Reference Ventura, Gomes, Pita, Neto and Taylor2013; Barona et al., Reference Barona, Kothari, Skuse and Micali2015).
The present study suggests a Trivers-Willard effect of parental income on the masculinization/feminization (as measured by 2D:4D) of their children such that high-income mothers masculinize their children in utero and low-income mothers prenatally feminize their children. Therefore, parental income should be negatively related to the 2D:4D of their children. This prediction is tested in a large online survey (the BBC Internet Study).
Methods
The BBC Internet Study was a multi-ethnic and multi-national survey, hosted by the BBC Science and Nature website in July 2005. It comprised around 200 questions concerning cognitive and behavioural tests and included information on demographics, personality, sexual behaviour and physical characteristics, such as 2D:4D (Reimers, Reference Reimers2007). A sample of 255,116 participants completed all study tasks. In addition to ethnicity (Asian/Asian British, Black/Black British, Black other, Chinese, Middle/Near Eastern, Mixed Ethnic, White), participants provided information about their age (integer 0 to 99 years), gender (male or female) and where they lived (the United Kingdom, then 240 other countries). The predominant ethnicity was White (reported by 84.1% of participants), and the most commonly represented nationalities were the United Kingdom (46.9%), the United States (27.7%), Canada (5.2%) and Australia (3.6%), with eleven other nations represented by >1000 participants.
Participants responded to a single question item concerning their parents’ income. The item was phrased ‘What best describes your parents’ income [while growing up],’ with response options: Group I=much lower than others (bottom 25% of the population), Group II=slightly lower than others (low 50% of the population), Group III=slightly higher than others (upper 50% of the population) and Group IV=much higher than others (top 25% of the population).
Participants self-measured 2D and 4D of their right and left hands using the methodology of Manning et al. (Reference Manning, Scutt, Wilson and Lewis-Jones1998). They viewed a diagram of the hand and were instructed to measure their fingers on the ventral side of the digit from the fingertip to the most proximal crease with a conventional ruler. Measurements were reported to the nearest millimetre using dropdown menus. The 2D:4D was calculated by dividing the 2D by 4D digit lengths. In the present study, the analyses were restricted to participants 18 years and older. As in earlier reports, the tails of the 2D:4D distributions were removed by considering right and left 2D:4D within the range of ≥0.80 to ≤1.20.
Results
Descriptive statistics
There were 189,318 participants (99,672 males). The numbers of male and female participants in each parental income group are reported in Table 1. Mean (SD) male 2D:4D varied from 0.985 (0.051) right and 0.986 (0.049) left in Group I down to 0.982 (0.049) right and 0.983 (0.048) left for Group IV. For females, the respective variation was from 0.995 (0.052) right and 0.993 (0.050) left for income Group I down to 0.993 (0.050) right and 0.991 (0.050) left for Group IV (see Table 1 and Figure 1).
Table 1. Means and SDs for 2D:4D by sex and hand by parental income groups for all participants, White participants, and UK and US participants
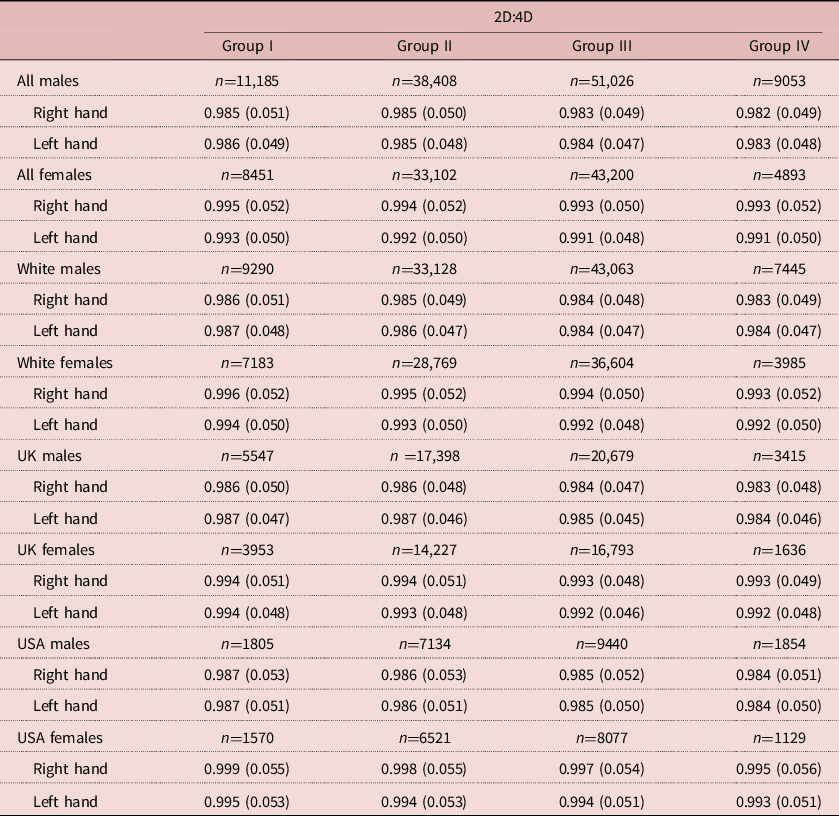
Income groups: I=bottom 25%, II=low 50%, III=upper 50% and IV=top 25%.
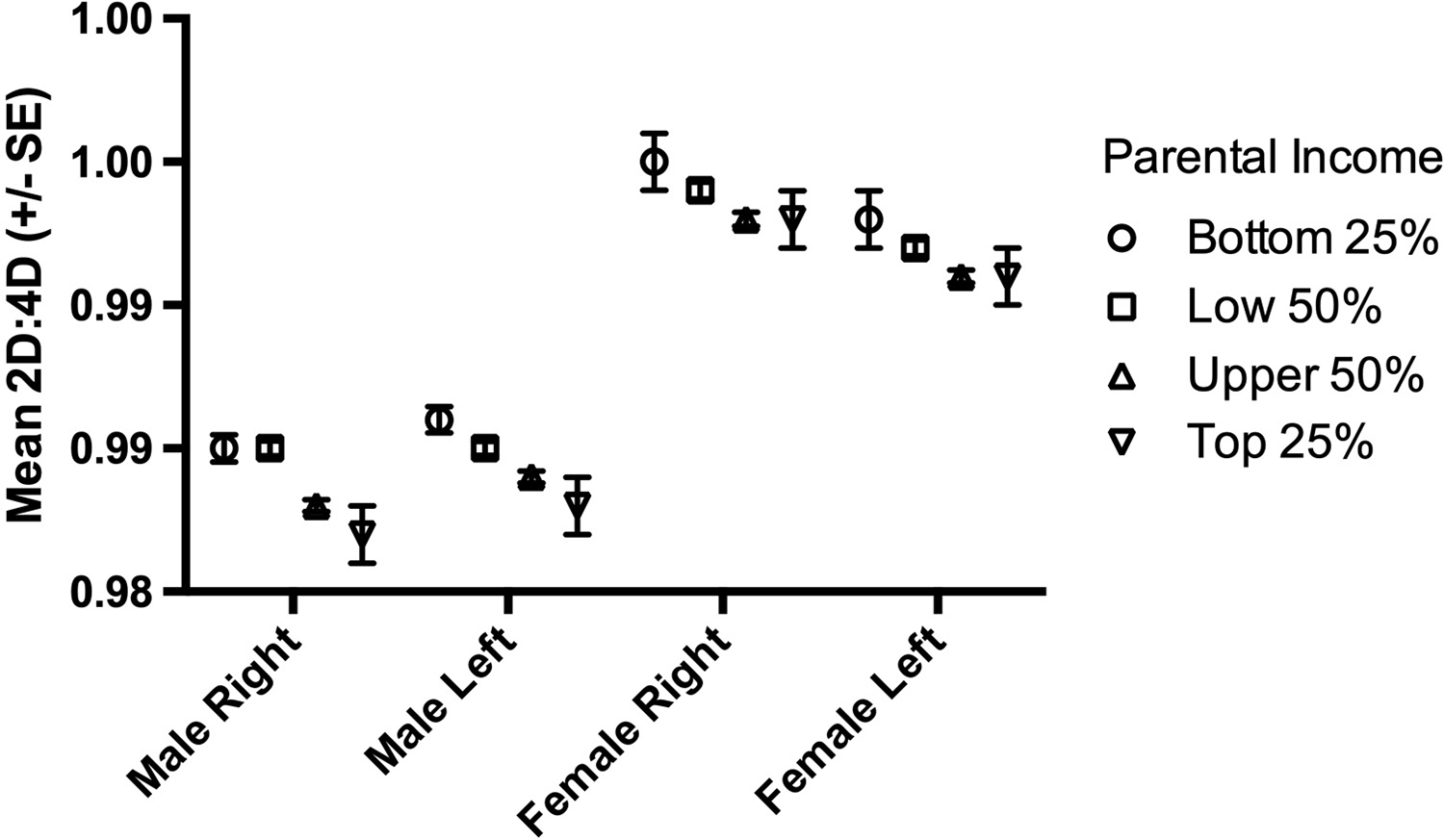
Figure 1. The relationship between parental income and mean right and left hand 2D:4D in males and females for all participants.
Analyses for the total sample
In males, parental income showed an effect on both right and left 2D:4D (right hand F (3,109668)=12.55, p<0.0001; left hand F (3,109668)=14.86, p<0.0001). Post-hoc tests (Fisher’s PLSD) indicated differences for both right and left hand 2D:4D for all pairwise comparisons between parental income groups below and above population average (2D:4D below-average income > 2D:4D above-average income) with mean differences I–III=0.002, I–IV=0.003, II–III=0.002 and II–IV= 0.002 (all p<0.0001). There were no differences in right or left 2D:4D for the pairwise comparisons between parental income groups below population average (I–II) or above population average (III–IV) (all p>0.05; Figure 1).
In females, parental income showed an effect on both right and left hand 2D:4D (right hand F (3,89642)=4.74, p=0.003; left hand F (3,89642)=4.02, p=0.007). These effects were weaker than for males. There were differences (Fisher’s PLSD) for right hand 2D:4D for only two pairwise comparisons between parental income groups with mean differences I–III=0.002 (p=0.006) and II–III=0.001 (p=0.002). For left hand 2D:4D there were three significant pairwise comparisons with mean differences I–III=0.002 (p=0.003), I–IV=0.002 (p=0.03) and II–III=0.001 (p=0.02). All significant pairwise comparisons showed offspring 2D:4D from below population average parental income groups were greater than offspring 2D:4D from above population average parental income groups (Table 1, Figure 1).
Thus far the analyses considered a multi-ethnic sample. However, 2D:4D varies across ethnic groups. Splitting the sample by ethnicity resulted in smaller groups and a consequent reduction in power. Therefore, the following analyses considered patterns of offspring 2D:4D across parental income groups in the numerically largest ethnicity in the BBC Internet Study, i.e. Whites.
White participants
There were 169,467 White participants in the analyses. The numbers of male and female participants in each parental income group are reported in Table 1. The pattern for White participants showed high 2D:4D in income Group I and low 2D:4D in income Group IV, e.g. for the right hand, Group I males 0.986 (0.051), females 0.996 (0.052); Group IV males 0.983 (0.048), females 0.993 (0.052) (see Figure 2).
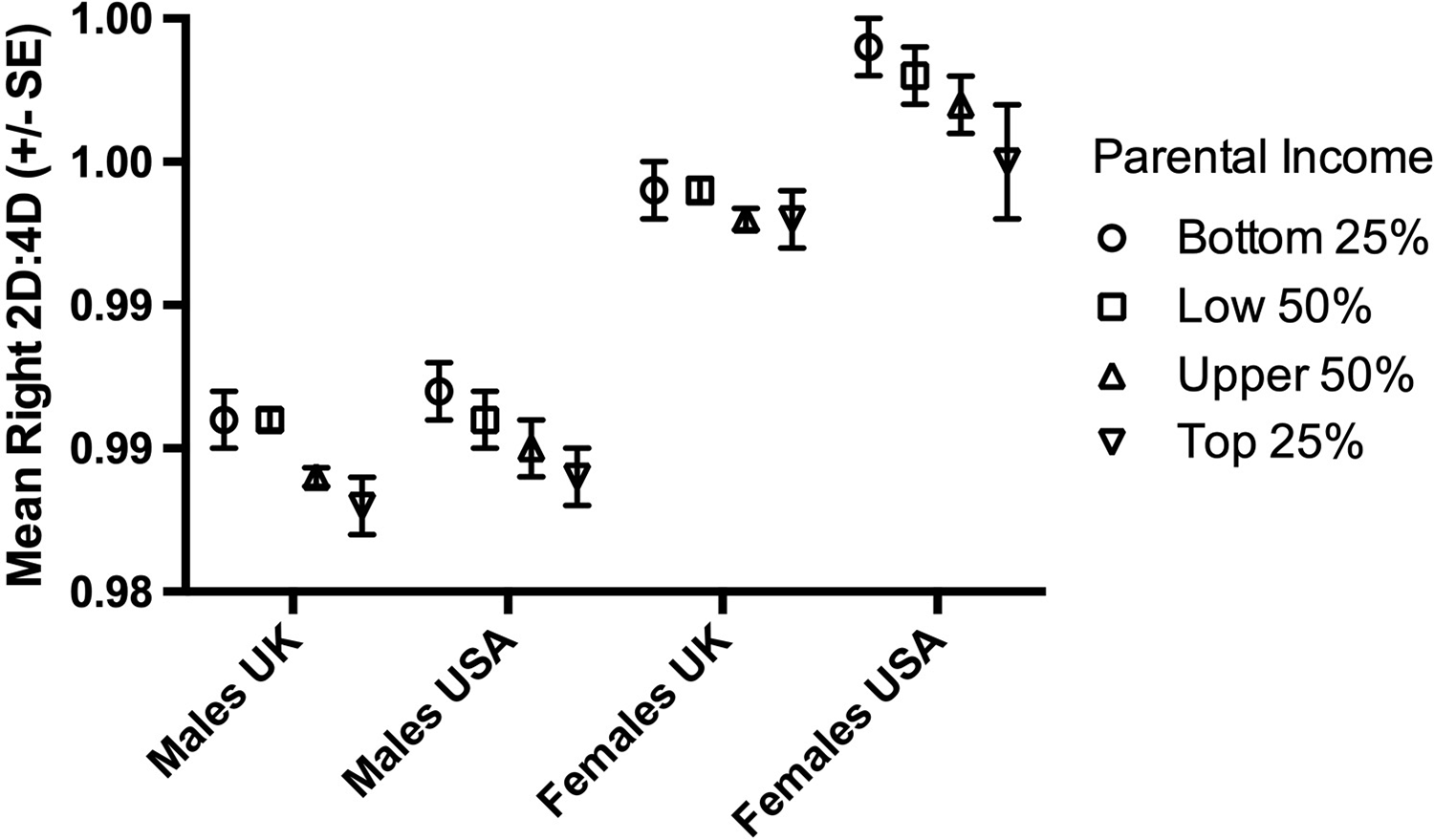
Figure 2. The relationship between parental income and mean right hand 2D:4D in White participants by nation (UK and USA) for males and females.
Analyses for White participants
Parental income had an effect on both the male right and left hands (right hand F (3,109668)=12.55, p<0.0001; left hand F (3,109668)=14.86, p<0.0001). Post-hoc tests (Fisher’s PLSD) showed similar patterns to those found in the total sample. There were differences for both right and left hand 2D:4D in four pairwise comparisons between parental income groups below and above population average (2D:4D below-average income > 2D:4D above-average income), with mean differences I–III=0.002 (p=0.0002) for right hand and 0.002 (p<0.0001) for left hand, I–IV=0.003 (p<0.0001) for both hands, II–III=0.002 (p<0.0001) for both hands and below II–IV=0.003 (p<0.0001) for both hands. There were no differences in right or left 2D:4D for the pairwise comparisons between parental income groups below population average, I–II or above population average III–IV (all p>0.05).
In White female participants, parental income showed an effect for both right and left hand 2D:4D (right hand F (3,76537)=5.14, p=0.002; left hand F (3,76537)=4.01, p=0.007). These effects were weaker than for males. There were differences (Fisher’s PLSD) for 2D:4D for only three pairwise comparisons between parental income groups with mean differences I–III=0.002 (p=0.004 and p=0.008 for right and left hands, respectively), II–IV=0.002 (p=0.03 and p=0.02 for right and left hands, respectively) and II–III=0.001 (p=0.002 and p=0.01 right and left, respectively). Pairwise comparisons I–II, II–IV and III–IV were not significant (all p<0.05). All significant pairwise comparisons showed offspring 2D:4D from below population average parental income groups were greater than offspring 2D:4D from above-average parental income groups.
White participants in the UK and USA
To account for the effect of nation, two-way ANOVAs were performed for males and females with the independent variables parental income and the nations in the study with the largest White representation (UK and the USA) and the dependent variable 2D:4D.
For males, there were main effects for parental income for both the right hand (F (3,67264)=9.62, p<0.0001) and left hand (F (3,67264)=10.11, p<0.0001) 2D:4D but no effects of nation (right hand F (1,67264)=1.89, p=0.17; left hand F (1,67264)=0.008, p=0.93). There were no interaction effects (all p>0.05). Overall, male mean 2D:4D reduced with increasing income but there were no differences between the mean 2D:4Ds of UK and USA participants (Figure 2).
For females, there was a main effect of parental income for the right hand (F (3,53898)=4.18, p=0.006) but not the left hand (F (3,53898)=1.52, p=0.21). Nation showed effects for both right (F (1,53898)=28.15, p<0.0001) and left hands (F (1,53898)=5.85, p=0.02). There were no interaction effects (all p>0.05). Overall, female mean 2D:4D reduced with increasing income but the effect was smaller than that seen for males. Mean 2D:4D was lower in UK participants than in participants from the USA (see Figure 2).
Discussion
The present study found that parental income affects children’s 2D:4D such that below-average income is related to high 2D:4D (feminization of the fetus) and above-average parental income is associated with low 2D:4D (masculinization of the fetus). These findings applied to the total study sample, the most numerous ethnic group in the study (i.e. Whites) and the most numerous national samples (UK and USA). Regarding the total sample, for male children, the effect was present for right and left 2D:4D and for all pairwise comparisons between parental income groups above and below the population average. For female children, the effect was also present on right and left hand 2D:4D and was found in pairwise comparisons between parental income groups above and below the population average. However, the female effects were weaker than the male effects, with only two significant pairwise comparisons for right hand 2D:4D and three for left hand 2D:4D.
The present findings are consistent with the Trivers-Willard hypothesis concerning maternal resources and its links to the influence of the mother’s sex steroids on fetal 2D:4D. Thus, mothers with high income will secrete elevated levels of T relative to E during the 1st trimester of their pregnancy, i.e. they will masculinize their male and female children. In contrast, women with low income will secrete low levels of T:E in the early stages of pregnancy. This hormonal milieu will feminize their male and female children. That is, high-income mothers will increase the fitness of their sons at the expense of their daughters while low-income mothers will increase the fitness of their daughters at the expense of their sons. Thus, there will be sexually antagonistic effects on the children of both high- and low-income mothers (Manning et al., Reference Manning, Barley, Walton, Lewis-Jones, Trivers and Singh2000). There is evidence for an effect of female condition on the production of sex steroids and sexually antagonistic effects of maternal sex steroids on the developing fetus.
There is only weak support for a link between the 2D:4D of women selected at random from the population and their production of T and E (Muller et al., Reference Muller, Giles, Bassett, Morris, Manning and Hopper2011). However, in contrast to women with low income, high-income women may benefit from high levels of nutrition. There may be associations between high income, good nutrition and the production of androgens in women. Elite women athletes with high standards of nutrition and in good condition (with high lean mass and low body fat levels) show negative relationships between their 2D:4D and the breakdown products of T (Eklund et al., Reference Eklund, Ekström, Thörngren, Ericsson, Berglund and Hirschberg2020) or salivary levels of T (Crewther & Cook, Reference Crewther and Cook2019). It appears that women in good condition can, and probably do, secrete elevated levels of T, particularly if they have low 2D:4D.
The Trivers-Willard hypothesis was originally formulated in the context of maternal manipulation of the sex ratio of progeny. There is indeed evidence that masculinized women (with high WHR and/or low 2D:4D) have more sons than feminized women (low WHR and/or high 2D:4D). This may be the result of the deleterious effects of high prenatal T on female fetuses and/or the advantageous effect of high prenatal T on male fetuses (Manning et al., Reference Manning, Anderton and Washington1996; Singh & Zambarano, Reference Singh and Zambarano1997; Manning & Bundred, Reference Manning and Bundred2001; Kim et al., Reference Kim, Oh, Kim, Yoon and Kim2015).
Maternal manipulation of the prenatal sex steroid environment of their children is likely to have later-life health consequences. For example, male children of low-income mothers will be feminized and more prone to several diseases. Prominent among these is likely to be the poverty influenced male-biased burden of cardiovascular diseases. A low income level has been consistently associated with cardiovascular disease, especially in high-income countries. In addition, disparities based on sex (males>females) have been shown in several studies. High 2D:4D in men has been linked to poor outcomes for cardiovascular disease such as early myocardial infarction, high blood pressure, atherosclerotic plaque development, high fibrinogen levels and markers of obesity (Manning & Bundred, Reference Manning and Bundred2001; Fink et al., Reference Fink, Manning and Neave2006; Lu et al., Reference Lu, Huo, Zhang, Wei, Shi and Peng2008, Reference Lu, Ma, Zhao and Huo2015; Ozdogmus et al., Reference Ozdogmus, Cakmak, Coskun, Verimli, Cavdar and Uzun2010; Kyriakidis et al., Reference Kyriakidis, Papaioannidou, Pantelidou, Kalles and Gemitzis2010; Wu et al., Reference Wu, Yang, Chai, Jin, Zhou, Peng and Zhao2013; Manning et al., Reference Manning, Bundred, Kasielska-Trojan, Smith-Straney and Mason2019; Bagepally et al., Reference Bagepally, Majumder and Kotadiya2020). Associated with all of these factors is a high level of parental poverty (Kucharska-Newton et al., Reference Kucharska-Newton, Harald, Rosamond, Rose, Rea and Salomaa2011; Mosquera et al., Reference Mosquera, San Sebastian, Waenerlund, Ivarsson, Weinehall and Gustafsson2016).
One possible limitation of the present study is that estimates of parental income in the early years of the family are dependent on their children’s recall. Inaccuracies that may result from faulty recall are likely to reduce the Trivers-Willard influence on 2D:4D. Thus, the reported effects may be conservative estimates of maternal influence on offspring 2D:4D. In order to minimize the recall effects of children’s estimates of parental income it is suggested that future studies should also include parental reports of family income.
In conclusion, inequality in parental income may be associated with the 2D:4D of their offspring. Children of parents of above-average income had low 2D:4D (high prenatal T:E) while the children of parents of below-average income had high 2D:4D (low prenatal T:E). The differences in offspring 2D:4D across income groups may arise because of maternal manipulation of sex steroids. Interpreting the findings of the present study through the lens of the Trivers-Willard hypothesis suggests that high-income mothers may masculinize their sons via increased levels of prenatal T. Male reproductive success shows higher variance than female reproductive success. Therefore, the fitness rewards from the sons of high-income mothers are likely to outweigh the deleterious effects of T on the daughters of high-income mothers. In contrast, mothers with low income are expected to feminize their children via increases in prenatal E. The fitness gain from feminized daughters is likely to outweigh the fitness loss of feminized sons. Moreover, the health costs of maternal manipulation of prenatal sex steroids may include hypertension, cardiovascular disease, high levels of fibrinogen and early myocardial infarction and could be focused on the feminized (low T, high E) sons of low-income mothers.
Funding
This research received no specific grant from any funding agency, commercial entity, or not-for-profit organization.
Conflict of Interest
The authors have no conflicts of interest to declare.
Ethical Approval
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.





