Calves are born agammaglobulinaemic, and are dependent on the timely consumption of maternal colostrum in sufficient volume and quality to confer immunity in the first few weeks of life through passive transfer (Godden et al., Reference Godden, Lombard and Woolums2019). Unfortunately newborn calves do not always have access to their dam's colostrum, either because of multiple births, acute mastitis or maladapted maternal behaviour, especially in first lactation heifers (Wereme et al., Reference Wereme, Strabel, Grongnet and Piot2001). Shortages in colostrum may also be precipitated by deliberate discarding of colostrum from cows infected with Mycobacterium avium subsp paratuberculosis and Mycoplasma bovis (McGuirk and Collins, Reference McGuirk and Collins2004).
According to published literature, 90% of Irish dairy producers store colostrum, while colostrum is routinely stored on 89% of large dairy farms in North America (Cummins et al., Reference Cummins, Berry, Murphy, Lorenz and Kennedy2017). Data on colostrum storage in the UK is limited, but recent survey data from Scottish farms found that 24/35 (68.6%) of farms stored colostrum and 22/24 (91.7%) of these used freezers to store colostrum (Haggerty et al., Reference Haggerty, Mason, Ellis and Denholm2021).
In the UK, colostrum is often harvested and fed to calves later, often being left in uncovered buckets at room temperature for extended periods (Haggerty et al., Reference Haggerty, Mason, Ellis and Denholm2021). Bacterial species double in number every 30 min at room temperature (21°C) and as such unpreserved colostrum feeding to neonatal calves should not be delayed (Stewart et al., Reference Stewart, Godden, Bey, Rapnicki, Fetrow, Farnsworth, Scanlon, Arnold, Clow, Mueller and Ferrouillet2005). A high proportion (36–42%) of individual colostrum samples exceeded TBC thresholds (>100 000 CFU/ml) in international literature (Fecteau et al., Reference Fecteau, Baillargeon, Higgins, Paré and Fortin2002; Morrill et al., Reference Morrill, Conrad, Lago, Campbell, Quigley and Tyler2012; Phipps et al., Reference Phipps, Beggs, Murray, Mansell, Stevenson and Pyman2016), while approximately 90% of pooled colostrum samples were highly contaminated (Denholm et al., Reference Denholm, Hunnam, Cuttance and McDougall2017b). McAloon et al. (Reference McAloon, Doherty, Donlon, Lorenz, Meade, O'Grady and Whyte2016) demonstrated that 56% of colostrum samples collected from Irish dairy farms were above the standard TBC and TCC thresholds, while in Scottish samples 31% and 27% failed to meet TBC and TCC thresholds, respectively (Haggerty et al., Reference Haggerty, Mason, Ellis and Denholm2021). This is comparable to estimates from Canadian dairy herds where 36% of samples exceeded TBC thresholds (Fecteau et al., Reference Fecteau, Baillargeon, Higgins, Paré and Fortin2002). Bacterial contamination comes from the udder, milking equipment, storage and feeding equipment (Donahue et al., Reference Donahue, Godden, Bey, Wells, Oakes, Sreevatsan, Stabel and Fetrow2012; Godden et al., Reference Godden, Lombard and Woolums2019). Every effort should be made by producers to minimise bacterial contamination of colostrum through scrupulous hygiene practices, including cleaning of cows' teats, thorough scrubbing of buckets and feeders with hot water and use of a detergent to break down the fatty residues deposited by colostrum. Some farmers also use sterile bags to collect and store colostrum and these may also be pasteurised (https://dairytechinc.com/perfect-udder).
Coliform species in particular have been shown to impair IgG absorption (Gelsinger et al., Reference Gelsinger, Jones and Heinrichs2015), acting through a number of mechanisms (Johnson et al., Reference Johnson, Godden, Molitor, Ames and Hagman2007). Firstly, physical binding of the IgG by microbes within the gastrointestinal lumen blocks their uptake across the enterocytes. Secondly, pathogenic bacteria may attach and damage intestinal cells meaning that their permeability is reduced. Thirdly, when these pathogenic bacteria damage intestinal cells there is accelerated gut closure. Fourthly, bacteria physically block absorption channels of the immunoglobulin molecules (Corley et al., Reference Corley, Staley, Bush and Jones1977; James et al., Reference James, Polan and Cummins1981; Staley and Bush, Reference Staley and Bush1985). Bacterial contamination could also include specific disease-causing calf pathogens such as E.coli, Salmonella species, Mycoplasma species or Mycobacterium avium paratuberculosis (Stewart et al., Reference Stewart, Godden, Bey, Rapnicki, Fetrow, Farnsworth, Scanlon, Arnold, Clow, Mueller and Ferrouillet2005; McAloon et al., Reference McAloon, Doherty, Donlon, Lorenz, Meade, O'Grady and Whyte2016).
If there is an absolute need to leave colostrum or milk out for prolonged periods at ambient temperatures or if bacterial counts are high (as they have been shown to be) then there is a place for some sort of colostrum preservative. Colostrum preservatives may also act to minimise the decline in IgG concentration in colostrum with time (Denholm et al., Reference Denholm, Hunnam, Cuttance and McDougall2017a), but the mechanism by which this occurs has not been established. The aim of this scoping review article was to identify options for preservation and gaps in research and to propose best practice for colostrum preservation.
Measures of preserved colostrum quality
Measures of performance for colostrum preservation include colostrum composition (focusing on fat and protein), immunoglobulin concentration (IgG >50 g/l), bacterial counts (<100 000 CFU/ml TBC and <10 000 CFU/ml coliforms), pH, serum IgG concentrations in calves (IgG >10 g/l), calf morbidity (<10%) and mortality (<2%), palatability and average daily gains (>0.9 kg/calf/day).
Colostrum pH and acidification
Normal pH of colostrum is 5.59–6.42 (Stewart et al., Reference Stewart, Godden, Bey, Rapnicki, Fetrow, Farnsworth, Scanlon, Arnold, Clow, Mueller and Ferrouillet2005; Cummins et al., Reference Cummins, Berry, Murphy, Lorenz and Kennedy2017; Hyrslova et al., Reference Hyrslova, Krausova, Michlova, Kana and Curda2020). Lowering the pH of colostrum is thought to inhibit microbial proliferation, however, most of the work on manual acidification by chemical additives has used milk or milk replacer, rather than colostrum. Early work by Wheeler et al. (Reference Wheeler, Ikurior and Stone1980) showed that the palatability of colostrum was negatively influenced by increasing concentration of acid preservative. Calves refuse more milk replacer preserved at pH 4.2 than at 5.2, since low pH colostrum and milk is unpalatable (Hill et al., Reference Hill, Bateman, Aldrich, Quigley and Schlotterbeck2013). Collings et al. (Reference Collings, Proudfoot and Veira2011) demonstrated rejection of milk replacer acidified to pH 4.3–4.4, however calves still seemed motivated to suck acidified milk (Todd et al., Reference Todd, Millman, Leslie, Anderson, Sargeant and DeVries2018).
Todd et al. (Reference Todd, Leslie, Millman, Sargeant, Migdal, Shore, Anderson and DeVries2016) also showed that milk replacer acidification tended to be associated with earlier solid feed consumption (presumably due to a palatability issue with the acidified liquid feed), whilst Coelho et al. (Reference Coelho, Tomaluski, Donde, Toledo, Bernardes, Jeronymo, Junior, Silva, Reis and Bittar2020) on the other hand showed no effect on feed intake when acidified milk, milk replacer and whole milk were compared. It is worth noting that in the same study, feeding acidified milk negatively affected calf weight gain compared with whole milk, however, in other work, calves fed acidified milk and non-acidified milk did not show any differences in average daily gain (Ribeiro et al., Reference Ribeiro, Pereira, de Queiroz, Cecon, Detmann and Gomes Azevêdo2009; Hill et al., Reference Hill, Bateman, Aldrich, Quigley and Schlotterbeck2013). Acidified milk has also been reported to increase the incidence of alopecia and diarrhoea in calves (Campos et al., Reference Campos, Scatamburlo and Rodrigues1986).
Previous research documented a reduction in immunoglobulin absorption in calves fed colostrum of low pH (pH = 4.65; Foley and Otterby, Reference Foley and Otterby1978), but a more recent study suggested that a pH as low as 5.0 did not affect the absorption of IgG in calves (Quigley et al., Reference Quigley, French and James2000). It has also been shown that colostral total bacteria counts (TBC) were negatively correlated with pH (Pearson r = −0.87), indicating that a greater TBC was associated with a lower pH (Cummins et al., Reference Cummins, Berry, Murphy, Lorenz and Kennedy2017).
Separation of milk and colostrum occurs as pH is lowered to 4.2 and gentle agitation is needed to re-homogenise milk. There is little evidence that acidification affects nutrients in milk or milk replacer or utilisation of these by the calf. A balance must be struck as if pH is too low calves will not drink and, if pH is too high, the milk will not be preserved leading to spoilage.
What is colostrum preserved with?
Colostrum may be preserved by the addition of chemical preservatives, low temperatures (freezing and refrigeration) or by addition of bacterial cultures. Colostrum may also be preserved by ‘natural’ aerobic or anaerobic fermentation. Low temperatures and low pH have been shown to slow bacterial growth (Stewart et al., Reference Stewart, Godden, Bey, Rapnicki, Fetrow, Farnsworth, Scanlon, Arnold, Clow, Mueller and Ferrouillet2005). Mycoplasma species can survive at pH in excess of 5 and Salmonella and Mycobacterium avium paratuberculosis (MAP) at pH in excess of 6 and 7 respectively. Optimal pH for growth of various pathogenic bacterial species (including Escherichia coli, Clostridia sp. and Salmonella sp.) range from 6 to 7.5 (Anderson, Reference Anderson2008).
Preserving colostrum using chemical additives: general
Acid preservatives present a number of safety concerns. Some acids are available in powdered form making them easier to handle than caustic liquids. However, dust can irritate the eyes, nose and throat. Dry products will also absorb moisture so need to be kept in an airtight container, which has practical implications for on-farm storage. Gloves, protective goggles and long sleeves are recommended as well as careful handling and immediate hand washing.
Numerous acids have been tested in colostrum and in cheese making to limit microbial growth. Acids can be short-chain organic acids including citric, acetic, formic, propionic and lactic acids. This approach may be complemented by the addition of low concentrations of specific lipid-soluble weak acids, for example, benzoic and sorbic acids. The combined effect of a low pH plus a high weak-acid concentration leads to acidification of the cytoplasm, which is usually sufficient to restrict microbial growth, but may also have other specific effects on cell activity (Booth and Stratford, Reference Booth, Stratford, Russell and Gould2003). Acidification of colostrum may be problematic due to the decomposition of lactose, which reduces digestibility. Puppel et al. (Reference Puppel, Golebiewski, Grodkowski, Slosarz, Kunowska-Slosarz, Solarczyk, Lukasiewicz, Balcerak and Przysucha2019) showed that the absorbability of all colostral elements of acidified colostrum is reduced (in comparison with fresh colostrum). IgG absorption is also depressed in an acidic environment as the mechanism of non-selective pinocytosis by which IgG is transported across the intestinal epithelium is pH-dependent (Heinrichs and Elizondo-Salazar, Reference Heinrichs and Elizondo-Salazar2009). Acid tolerant yeasts and moulds may contribute to poor palatability of colostrum and degradation of nutrients (Drevjany et al., Reference Drevjany, Irvine and Hooper1980).
Many of the trials conducted in the 1970s and 1980s advocated dilution of acidified colostrum with water, which adversely affects calf growth rates by diluting the nutrients in the feed. The efficiency of feeding pasteurised and acidified waste milk were comparable in some work, and the acidification of waste milk was deemed an acceptable labour-saving and diarrhoea-preventing feed for young calves (Zou et al., Reference Zou, Wang, Deng, Cao, Li and Wang2017).
Preserving colostrum using chemical additives: citric acid
Although citric acid is a well-recognised preservative in food, the effectiveness of citric acid as a preservative in feeding stuffs and water for drinking has not been sufficiently demonstrated (Matsuda et al., Reference Matsuda, Yano, Maruyama and Kumagai1994). Inhibition of a wide range of bacteria and fungi occurred only at concentrations above 25 000 mg citric acid/L, which are greater than the recommended-use concentration of citric acid in feed and corresponding concentration in water for drinking (European Food Safety authority: EFSA). Citric acid is safe according to USFDA (United States Food and Drug Administration) and EFSA (EFSA Feedap Panel, 2015) and can be used legally without restriction in the USA at rates of 15 000 mg/kg in feed and 5000 mg/l in water.
Canning et al., Reference Canning, McIntyre and Anderson2009 added citric acid to whole milk and pH was maintained at 4.5 for about 4 d. In addition to the antimicrobial effect of citric acid (by lowering pH), studies have indicated that the chelating effect of citric acid also inhibits bacteria. By chelating or binding metal ions, the substrate for bacterial growth is diminished in the food, thus influencing growth (Søltoft-Jensen and Hansen, Reference Søltoft-Jensen and Hansen2005). The New Zealand livestock industry has been concerned with the eradication of Mycoplasma species (sp.), first identified in New Zealand in 2017. There are a number of practical guidelines developed by New Zealand industry bodies (Beef and Lamb NZ and DairyNZ) on the acidification of milk using citric acid to mitigate Mycoplasma sp. (see online Supplementary File Table S1).
Preserving colostrum using chemical additives: propionic acid
Using propionic acid (available in liquid form) to acidify milk at a concentration of 1% and a rate of 35–40 ml/gallon resulted in a variation in pH of milk from 4.1 to 5. Milk acidified with propionic acid was not well accepted by calves as it has a pungent, rancid odour. There are safety concerns for liquid propionic acid, including burning of the skin and irritation of mucous membranes. The acid is also corrosive to most metals. Despite this, propionic acid is safe (according to USFDA and EFSA) and can be used legally without restriction in the USA and at rates of 10–30 g/kg in feed.
Muller and Syhre (Reference Muller and Syhre1975) found that propionic acid maintained pH after 23 d of fermentation, in comparison with lactic acid and 3 bacterial cultures (Streptococcus lactis, Streptococcus tberrnopbilus, and Lactobacillus bulgaricus, 1%). Jenny et al. (Reference Jenny, Hodge, O'Dell and Ellers1984) compared sodium benzoate, propionic acid and formaldehyde as preservatives for colostrum and found that titratable acidity was highest for propionic acid preserved colostrum, with potential detrimental effects on palatability. In addition, first milking colostrum preserved with 1% propionic acid or 0.3% formic acid and stored for 4 weeks had lower IgG concentrations than aerobically fermented or frozen (−4°C) colostrum (Schipper et al., Reference Schipper, Kotta, Staples, Fisher and Eriksen1981).
Rindsig and Bodoh (Reference Rindsig and Bodoh1977) observed more refusals of liquid diets by calves fed colostrum treated with propionic acid than when calves were fed whole milk, naturally aerobically fermented colostrum or colostrum treated with formaldehyde. Refusals were attributed to a combination of odour, taste and low pH. Conversely, Polzin et al. (Reference Polzin, Otterby and Johnson1977) observed no refusals of colostrum containing propionic or formic acids.
Preserving colostrum using chemical additives: formic acid
Formic acid is not currently approved by the USFDA due to skin and eye contact irritation and serious eye damage. Formic acid is volatile, and exposure via inhalation for those handling the additive is considered to present a risk to unprotected workers. Turnover of formic acid is, however, rapid with no evidence of accumulation in body tissues and use in animal nutrition is not expected to contribute to human exposure.
Formic acid is used as a preservative and antibacterial agent in livestock feed in the UK at a rate of 10 000 mg/kg complete feed following evaluation by the European Food Safety Authority (EFSA Feedap Panel, 2014). According to Canadian experience, preservation with formic acid (based on a Finnish model) could facilitate storage of milk or colostrum at room temperature. However, during warm seasons, refrigeration will ensure optimal preservation for up to 20 d (Anderson, Reference Anderson2008). There is some dispute as to necessary contact time for formic acid with some producers acidifying and feeding immediately, and others leaving milk for 6–12 h before feeding. Formic acid quickly kills coliforms in 1–2 h contact time. (Anderson, Reference Anderson2008). Formic acid also kills about 90% of MAP in 8 h contact time at pH 4.0 and 100% of MAP at 48 h (Mutharia and Raymond, Reference Mutharia and Raymond2007). Other acids (including hydrochloric and an orthophosphoric acid mix) vary in their effects on MAP with better results at 48 h contact time than 8 h contact time (Anderson, Reference Anderson2008).
It has been demonstrated that calves fed acidified waste milk (using formic acid) consumed more starter grain (potentially due to poor milk palatability) than calves fed untreated waste milk (Zou et al., Reference Zou, Wang, Deng, Cao, Li and Wang2017), but these animals did not have as high serum IgG concentrations and did not grow well. Acidification with formic acid (0.5 and 0.1%) did not lead to significant changes in crude protein or total solids in colostrum from Sahiwal cows after 28 d at ambient temperatures (Mbuthia et al., Reference Mbuthia, Gachuiri and Abate2002).
Finlanders stress the importance of using skim milk powder (rather than whey source milk powder) in their free-access formic acid acidified milk feeding systems, however, these are expensive in the UK and the amount of skim milk powder in the product is difficult to determine from product labelling. Anecdotally, feeding acidified milk preserved with formic acid resulted in fewer clinical cases of diarrhoea and fewer treatment interventions in milk fed calves (Anderson, Reference Anderson2008), however, palatability and safety issues have led some researchers to declare that formic acid is not a practical preservation agent for colostrum (Collings et al., Reference Collings, Proudfoot and Veira2011).
Preserving colostrum using chemical additives: formaldehyde and hydrogen peroxide
Formaldehyde has been used historically as a preservative (Mbuthia et al., Reference Mbuthia, Klobasa, Gachuiri and Abate1997), but its carcinogenic properties mean it is no longer approved by the USFDA and while it may still be used in Europe (at concentrations of between 200 and 1000 mg/kg feed) its use is not encouraged. Hydrogen peroxide is similarly problematic.
Early research by Muller and Smallcomb (Reference Muller and Smallcomb1977) showed that 0.25% formaldehyde maintained original colostral pH for 18 d. Bush et al. (Reference Bush, McQueen and Nicholson1980) applied formalin and fermentation to extend the shelf life of colostrum and reported a slower reduction in pH (from 6.2 to 5.6) for 24 d (at ambient conditions of 20–26°C) at 0.1% formalin than untreated colostrum. Literature pertaining to the effects of this type of chemical preservative on colostrum immunoglobulins is not available (Borad and Singh, Reference Borad and Singh2018)
Preserving colostrum using chemical additives: potassium sorbate
Potassium sorbate has been used extensively as a ‘stabiliser’ in wine production. Unlike acid agents, potassium sorbate only limits bacterial growth in colostrum. Bey et al. (Reference Bey, Godden, Lillegaard, Stewart, Rapnicki, Fetrow and Farnsworth2007) found that in refrigerated colostrum, preservation with potassium sorbate (0.5% final solution) reduced bacteria counts initially (1 log difference vs. raw non-preserved colostrum), then delayed growth rate. Potassium sorbate is more effective at prohibiting growth of moulds and yeasts than acids. Potassium sorbate-preserved colostrum may last up to 7 d, preferably at refrigeration temperatures (4°C) (Stewart et al., Reference Stewart, Godden, Bey, Rapnicki, Fetrow, Farnsworth, Scanlon, Arnold, Clow, Mueller and Ferrouillet2005); although some work in seasonal calving systems demonstrated its effectiveness to maintain IgG concentration and minimise bacterial proliferation even at ambient temperatures (Denholm et al., Reference Denholm, Hunnam, Cuttance and McDougall2017a).
Potassium sorbate is available in powdered form and is generally recognised as safe by USFDA and the EFSA. It is added at a rate of 1% by volume of a 50% solution (EFSA safe concentration 11 mg/kg body weight). Potassium sorbate can also be used in conjunction with heat treatment but needs to be added afterwards to avoid curd formation during the heat treatment process. According to DairyNZ, potassium sorbate is not effective at elimination Mycoplasma sp. in colostrum in the ‘required time frame’, although proper referencing is not provided.
Drevjany et al., Reference Drevjany, Irvine and Hooper1980 showed that potassium sorbate treated colostrum (applied at day 4 to fermented colostrum) resulted in increased calf starter consumption and greater weight gains in warm temperatures. Colostrum also retained palatability through 21 d of storage with little surface mould growth compared with untreated colostrum. Effective antimicrobial threshold for potassium sorbate is pH 6.5 (Drevjany et al., Reference Drevjany, Irvine and Hooper1980).
Preserving colostrum using chemical additives: sodium benzoate
Sodium benzoate (benzoic acid) may be added to milk but at a maximum limit of 0.1%. Jenny et al. (Reference Jenny, Hodge, O'Dell and Ellers1984) added sodium benzoate at 0.5% with acceptable preservative results (milk pH held at 5.1 for 10 d and 5.5 at 20°C or higher). The same study demonstrated that colostrum treated with sodium benzoate was slightly higher in fat and pH (due to buffering capacity) and lower in protein than other colostrum treatments (propionic acid and formaldehyde). In 1977 Muller and Smallcomb studied a number of chemicals: sodium benzoate (0.5%), sodium propionate, sodium formate, sodium acetate, benzoic acid, sorbitol, and gluconic acid lactone. Additions of sodium benzoate and benzoic acid resulted in a slower decrease in pH and maintenance of a more constant pH for 21 d than the control and colostrum with other additives. However, preservation with sodium benzoate altered physicochemical properties and destroyed nutritional components of colostrum (Borad and Singh, Reference Borad and Singh2018).
Preserving colostrum using low temperatures
According to some literature: ‘Chemical preservatives cannot preserve colostrum satisfactorily; chilling and freezing are the most preferred methods’ (Borad and Singh, Reference Borad and Singh2018). Warmer temperatures lead to proliferation of bacteria and highly contaminated colostrum resulted in lower serum IgG concentrations in calves (Elizondo-Salazar and Heinrichs, Reference Elizondo-Salazar and Heinrichs2009).
Morrill et al. (Reference Morrill, Conrad, Lago, Campbell, Quigley and Tyler2012) recommended that colostrum should be fed fresh from the dam or frozen immediately. Frozen colostrum (−20°C) may be stored for up to 1 year without affecting IgG concentration (Stewart et al., Reference Stewart, Godden, Bey, Rapnicki, Fetrow, Farnsworth, Scanlon, Arnold, Clow, Mueller and Ferrouillet2005). Proper labelling is recommended with cow identification number and date of collection, as well as storage in containers of no more than 2 l capacity to aid thawing (Robbers et al., Reference Robbers, Jorritsma, Nielen and Koets2021). Fresh or frozen first milking colostrum can be used to feed dairy calves, without the latter affecting the diversity in the colonisation of the intestinal tract. No significant differences in serum IgG concentration were observed between calves fed frozen and thawed colostrum and calves fed fresh colostrum (Holloway et al., Reference Holloway, Tyler, Lakritz, Carlson and Holle2001; Donovan et al., Reference Donovan, Reber, Gabbard, Aceves-Avila, Galland, Holbert, Ely and Hurley2007).
Colostrum should be thawed in a hot water bath heated to 40°C (Robbers et al., Reference Robbers, Jorritsma, Nielen and Koets2021). One should avoid microwaving frozen colostrum as this will create ‘hot pockets’ (>60°C) which may denature IgG molecules. A higher power of microwave has been associated with a loss of IgG, and heating above 60°C in a hot water bath resulted in a significant (26%) reduction in IgG1 (Balthazar et al., Reference Balthazar, Doligez, Leray and Cozler2015). Repeated freeze–thaw cycles will cause denaturation of colostrum IgG molecules, so a single thaw is advised. Compared with fresh colostrum, repeated freeze/thawing resulted in a significant decrease in IgG concentration of 7.8 and 7.7% for two and three freeze/thaw cycles, respectively (Robbers et al., Reference Robbers, Jorritsma, Nielen and Koets2021). A log reduction in Mycoplasma sp. through freezing has also been demonstrated (Gille et al., Reference Gille, Boyen, Van Driessche, Valgaeren, Haesebrouck, Deprez and Pardon2018).
Refrigeration (at 4°C) may be employed for short-term storage of colostrum, but colostrum stored in this way should be fed within 2 d of harvest (Cummins et al., Reference Cummins, Berry, Murphy, Lorenz and Kennedy2017). In this work colostrum stored at ambient temperatures (i.e., 22°C) had more than 42 times more bacteria present as well as pH 0.85 units lower and serum IgG concentration 2 times lower than colostrum stored at 4°C for 2 d (Cummins et al., Reference Cummins, Berry, Murphy, Lorenz and Kennedy2017). While colostrum stored at 4°C for 2 d had more bacteria present than pasteurised and fresh colostrum, this did not result in reduced calf serum IgG concentrations in this study. Langel et al. (Reference Langel, Wark, Garst, James, McGilliard, Petersson-Wolfe and Kanevsky-Mullarky2015) noted that refrigeration (4°C) up to 8 h did not affect cell viability, but effects of refrigeration for a longer period are yet unclear.
The main disadvantage to using refrigeration or freezing facilities to preserve colostrum is the associated capital cost and the space required. Furthermore, many farmers don't have or don't check thermometers on refrigerators and freezers or have broken equipment (poorly maintained, dirty) (Haggerty et al., Reference Haggerty, Mason, Ellis and Denholm2021).
Lactobacillus and yoghurt culture inoculations
Ellinger et al. (Reference Ellinger, Muller and Glantz1980) inoculated whole milk with Lactobacillus acidophilus and demonstrated a linear decrease in coliforms suggesting an antagonistic action towards coliforms. A similar effect has also been demonstrated in pigs (Muralidhara et al., Reference Muralidhara, Sheggeby, Elliker, England and Sandine1977). Lactobacillus acidophilus may be fed as viable cultures or as a dried preparation and has been shown to decrease the incidence of diarrhoeal disease in calves in some work, but not in others (Ellinger et al., Reference Ellinger, Muller and Glantz1980).
While it has been suggested that fermentation of bovine colostrum by suitable strains might be helpful in the prevention of diarrhoea in calves or to increase colostrum quality by inhibition of pathogenic and spoilage microbiota, a comparison of ‘Easiyo’ yoghurt cultures and untreated colostrum showed no difference in bacterial growth in pooled colostrum samples form seasonal calving herds (Denholm et al., Reference Denholm, Hunnam, Cuttance and McDougall2017a).
Bush et al. (Reference Bush, McQueen and Nicholson1980) found that 0.1% formalin was more effective in preserving colostrum than either Streptococcus lactis or yoghurt culture. Drevjany et al. (Reference Drevjany, Irvine and Hooper1975) reported that colostrum inoculated with Lactobacillus acidophilus was unacceptable to calves due to a pH of less than 4.0.
Fermentation: general
Fermentation may be an alternative to low temperature or chemical storage and may be aerobic or anaerobic. Fermentation causes the development of beneficial microorganisms, such as lactic acid bacteria, and the concomitant pH reduction preserves colostrum at room temperature (Otterby et al., Reference Otterby, Johnson, Foley, Tomnsche, Lundquist and Hanson1980).
Fermentation: aerobic fermentation
Much of the work from the late 1970s and early 1980s found that fermenting colostrum under aerobic conditions resulted in a rapid drop in pH particularly when colostrum was stored at higher temperatures (Muller and Syhre, Reference Muller and Syhre1975; Bush et al., Reference Bush, McQueen and Nicholson1980). Jenny et al. (Reference Jenny, O'Dell and Johnson1977) also reported a putrid odour and mould development when colostrum was stored at 27°C or at higher temperatures. This was corroborated by Rindsig and Bodoh (Reference Rindsig and Bodoh1977), when colostrum was stored at temperatures between 32 and 39°C. The authors suggested discarding colostrum under these conditions since its voluntary intake by calves was also low.
Carlson and Muller (Reference Carlson and Muller1977) showed that naturally fermented colostrum had more nutrient breakdown during storage than did 1% propionic acid treated, with formaldehyde (0.05%) treated colostrum intermediate. Aerobic bacteria counts (particularly coliform counts) were still high after 21 d of storage in some work (Thompson and Marth, Reference Thompson and Marth1976), discounting the theory that the fermentation process produces sufficient lactic acid to eliminate E. coli from colostrum so that the calf does not ingest these organisms in large numbers and hence does not develop scours (Thompson and Marth, Reference Thompson and Marth1976). Furthermore, it has been suggested in much of the published work that aerobically fermented colostrum should be fed diluted with water such as not to induce scouring (Thompson and Marth, Reference Thompson and Marth1976), which is inadvisable as previously mentioned.
Foley et al. (Reference Foley, Hunter and Otterby1978) went on to assert that aerobically fermented colostrum is a potential source of antibodies for newborn calves when maternal colostrum is not available, but it is difficult to form colostrum banks since storage periods are short. Feed costs were estimated to be reduced by 90% with a fermented colostrum feeding program compared with a whole milk feeding program (Yu et al., Reference Yu, Stone and Wilson1976).
Fermentation: anaerobic fermentation
Ferreira et al. (Reference Ferreira, Silva, Paula, Soares and Bittar2013) experimented with anaerobic fermentation, making ‘colostrum silage’ and found that the pH quickly decreased when ensiled colostrum was stored at higher temperatures (32.5°C). Their results indicated that the temperature at which colostrum was fermented directly influenced the speed and intensity of microbial population development and degradation of the main nutritional parameters, such as casein and lactose; although Saalfeld et al. (Reference Saalfeld, Pereira, Silveira, Schramm, Valente, Borchardt, Gularte and Leite2013) did not find such detrimental effects of higher temperatures.
Saalfeld et al. (Reference Saalfeld, Pereira, Silveira, Schramm, Valente, Borchardt, Gularte and Leite2013) stored colostrum in sealed bags at room temperature for 21 d. Physicochemical evaluation of colostrum silage revealed a tendency to maintain protein, dry matter and fat values, but lactose percentage decreased. pH of anaerobically fermented colostrum fell after 7 d of fermentation with a concurrent increase in lactic acid percentage, but ‘colostrum silage’ fed calves gained more weight than the control milk fed calves indicating that the drop in lactose in the anaerobically fermented colostrum was not detrimental to calf growth. The presence of the bacteria Lactobacillus, Staphylococcus, Escherichia, Klebsiella, Bacillus and Candida yeast species was observed in ‘colostrum silage’ for up to 14 d, but from 21 d of fermentation only bacteria of the genus Lactobacillus species were isolated. This indicated that the pH of the colostrum fermented anaerobically does not support the proliferation of pathogenic organisms which may otherwise have been transmitted via colostrum to calves (Stewart et al., Reference Stewart, Godden, Bey, Rapnicki, Fetrow, Farnsworth, Scanlon, Arnold, Clow, Mueller and Ferrouillet2005). Further work by Saalfeld et al. (Reference Saalfeld, Pereira, Borchardt, Sturbelle, Rosa, Guedes, Gularte and Leite2014) showed that colostrum immunoglobulin concentration was not compromised by anaerobic fermentation (compared with frozen colostrum) stored for 12 months and passive immunity was adequately transferred to newborn calves.
Anaerobically fermented colostrum may potentially be stored for much longer periods (up to 12 months) than aerobically fermented colostrum. Natural aerobic acidification, with and without preservatives, makes colostrum preservation feasible for only between 28 (Gonzáles et al., Reference Gonzáles, García and López1978) and 90 (Thompson and Marth, Reference Thompson and Marth1976) days.
Pasteurisation
While pasteurisation is not strictly speaking a method of preservation, it is a useful tool in storage and managing the shelf life of colostrum. As early as 1981, James et al. suggested that a greater bacterial concentration in the calf's gut may adversely affect the passive transfer of IgG. Numerous studies have demonstrated that heat treatment and consequent decreased bacterial counts in colostrum lead to improved immunity and weight gain in dairy calves (Johnson et al., Reference Johnson, Godden, Molitor, Ames and Hagman2007; Elizondo-Salazar and Heinrichs, Reference Elizondo-Salazar and Heinrichs2009; Gelsinger et al., Reference Gelsinger, Jones and Heinrichs2015). However, IgG molecules may be destroyed if colostrum is heated to greater than 60°C. This is because immunoglobulins are mono- or polymeric proteins, formed by two light and two heavy polypeptide chains which are connected by disulphide bonds into a Y-shaped particle (Puppel et al., Reference Puppel, Golebiewski, Grodkowski, Slosarz, Kunowska-Slosarz, Solarczyk, Lukasiewicz, Balcerak and Przysucha2019) and excessive heating leads to an initially reversible unfolding of this native structure, with loss of globular configuration, which can proceed further to irreversible denaturation and aggregation via hydrophobic and disulphide interactions (Indyk et al., Reference Indyk, Williams and Patel2008).
Cummins et al., Reference Cummins, Berry, Murphy, Lorenz and Kennedy2017 investigated the effects of colostrum, stored under various conditions, fed to Irish spring born calves and found that pasteurised colostrum resulted in serum IgG concentrations two times higher than colostrum stored in warm conditions (22°C). Pasteurisation also effectively destroys MAP, Salmonella and Mycoplasma species in milk deliberately spiked with these organisms (Stabel et al., Reference Stabel, Hurd, Calvente and Rosenbusch2004). Pasteurisation units are not commonplace on UK dairy farms due to the high capital cost involved.
Goat colostrum preservation
In some countries dairy goats are prevalent and international research has focused on colostrum additives for preservation. Spanish researchers found no difference in aerobic mesophilic bacteria counts between either 10 or 14% glycerol and propylene glycol additives. These additions reduced bacterial count to a greater extent than untreated colostrum, and 2 or 6% additions of these compounds. They concluded that glycerol addition to goat colostrum before heat treatment is suitable to enhance bacterial reduction (Morales-delaNuez et al., Reference Morales-delaNuez, Hernandez-Castellano, Moreno-Indias, Sanchez-Macias, Arguello and Castro2020).
Sodium dodecyl sulphate (1%) was found to be an efficient colostrum biocide that, unlike pasteurisation, does not affect immune passive transfer or goat kid health (Morales-delaNuez et al., Reference Morales-delaNuez, Moreno-Indias, Sánchez-Macías, Capote, Juste, Castro, Hernández-Castellano and Argüello2011). Neither of these compounds has been tested in bovine colostrum and this could be an area for further research.
New technologies for colostrum preservation for human consumption or for neonatal calves
Many of the following colostrum processing treatments would be difficult to practically perform on farm and are more suited to the processing of colostrum in a laboratory or controlled setting. They are included here for completeness and may be the future of on-farm colostrum preservation with advances in technology.
New technologies: UV light radiation
Teixeira et al. (Reference Teixeira, Bicalho, Machado, Oikonomou, Kacar, Foditsch, Young, Knauer, Nydam and Bicalho2013) found that IgG and lactoferrin concentrations were significantly lower in UV light treated colostrum than in raw colostrum, however, there were no significant differences in serum IgG concentrations among calves fed heat or UV treated or untreated colostrum. It is important to note that UV light treatment may not work as well in thick colostrum as in milk (Teixeira et al., Reference Teixeira, Bicalho, Machado, Oikonomou, Kacar, Foditsch, Young, Knauer, Nydam and Bicalho2013) and that the presence of dissolved and suspended solids can scatter UV light and provide a site for bacterial aggregation, attenuating the bactericidal activity of this form of radiation (Koutchma et al., Reference Koutchma, Keller, Chirtel and Parisi2004; Ye et al., Reference Ye, Koutchma, Parisi, Larkin and Forney2007). UV light radiation did not reduce bacterial counts as effectively as heat treatment (63°C for 6 min) and, for unknown reasons, resulted in a greater reduction in colostrum IgG concentrations (Teixeira et al., Reference Teixeira, Bicalho, Machado, Oikonomou, Kacar, Foditsch, Young, Knauer, Nydam and Bicalho2013). UV irradiation of milk spiked with MAP also did not result in an adequate reduction in infectivity (Donaghy et al., Reference Donaghy, Keyser, Johnston, Cilliers, Gouws and Rowe2009). Pereira et al. (Reference Pereira, Bicalho, Machado, Lima, Teixeira, Warnick and Bicalho2014) also studied the effect of UV light on colostrum IgG and bacterial contaminants and observed a negative linear relationship between duration UV treatment and IgG concentration. Puppel et al. (Reference Puppel, Golebiewski, Grodkowski, Slosarz, Kunowska-Slosarz, Solarczyk, Lukasiewicz, Balcerak and Przysucha2019) cite that preserving colostrum using UV irradiation, membrane filtration, pulsating electric field (PEF) and concentrated microwave fields (CMF) resulted in a number of changes in the chemical composition of the colostrum.
New technologies: Lyophilisation, spray drying or freeze drying
Lyophilisation (drying in a lower temperature and vacuum) has been shown to negatively impact colostral fat with consequent rapid spoilage. In addition, IgG absorption from lyophilised colostrum by the calf is 30% lower than fresh colostrum (Borad and Singh, Reference Borad and Singh2018).
Spray-drying produced a dried colostrum in which immunoglobulin quantity and function were preserved and was the most cost-effective at preserving the therapeutic potential of colostrum for human consumption (Chelack et al., Reference Chelack, Morley and Haines1993). Earlier investigations also showed that freeze-drying did not alter the concentration of immunoglobulins in colostrum (Klobasa et al., Reference Klobasa, Goel and Werhahn1998).
Spray drying is the most commonly applied technology for the manufacture of dairy powders and other ingredients, but concerns about heat-induced damage to colostrum proteins limited the adoption of spray drying for colostrum powder preparation since much of the IgG activity is destroyed.
Freeze-drying is the most preferred dehydration method for heat-sensitive biological material, as the low processing temperature and rapid local transition of frozen material from hydrated to dehydrated state minimises nutrient and immunoglobulin losses. Chelack et al. (Reference Chelack, Morley and Haines1993) reported a 10% loss in biological activity of immunoglobulins upon freeze-drying of colostrum, whereas Elfstrand et al. (Reference Elfstrand, Lindmark-Mansson, Paulson, Nyberg and Akesson2002) reported 34 and 25% losses in total immunoglobulins during freeze-drying of colostrum. Data from first milking postpartum colostrum samples from 18 Egyptian buffaloes and 36 Holstein cows showed that freeze dried colostrum stored at 7°C for 3 months had significantly reduced IgG concentrations compared with frozen colostrum (Abd El-Fattah et al., Reference Abd El-Fattah, Abd Rabo, El-Dieb and Satar El-Kashef2014).
A study by Bartkiene et al. (Reference Bartkiene, Lele, Sakiene, Zavistanaviciute, Ruzauskas, Stankevicius, Grigas, Pautienius, Bernatoniene, Jakstas, Zadeike, Viskelis and Juodeikiene2020) concluded that a combination of ultrasonication, fermentation, and dehydration could be used to reduce microbial contamination of bovine colostrum; however, more investigations are needed to evaluate the influence of these treatment methods on sensitive biologically active compounds in bovine colostrum.
New technologies: high pressure processing
Among novel technologies, high pressure processing has been found to be a promising preservation method for colostrum immunoglobulins (Borad and Singh, Reference Borad and Singh2018). High pressure processing retained 20% more bovine IgG in soy milk than heat treatment (at 75–78°C) (Li et al., Reference Li, Zhang, Balasubramaniam, Lee, Bomser, Schwartz and Dunne2006), but IgA molecules in human breast milk were destroyed by high pressure processing (Permanyer et al., Reference Permanyer, Castellote, Ramírez-Santana, Audí, Pérez-Cano, Castell, López-Sabater and Franch2010)
Masuda et al. (Reference Masuda, Rehinarudo, Suzuki, Sakai and Morichi2000) reported effective suppression of bacterial growth for 9 d at 4°C after treating colostrum at 300 and 400 megapascals (MPa) for 10 min. Up to 300 MPa, IgG remained intact, but application of 400 MPa resulted in altered viscosity of the colostrum and denaturation of IgG. Indyk et al. (Reference Indyk, Williams and Patel2008) and Foster et al. (Reference Foster, Poulsen, Sylvester, Jacob, Casulli and Farkas2016) found colostral IgG to be stable at treatments up to 400 MPa, as long as duration was limited to 30 min. Increasing pressure (500 or 600 MPa) or duration resulted in increased denaturation and aggregation.
Conclusion
Which preservation method is best for on farm preservation of bovine colostrum?
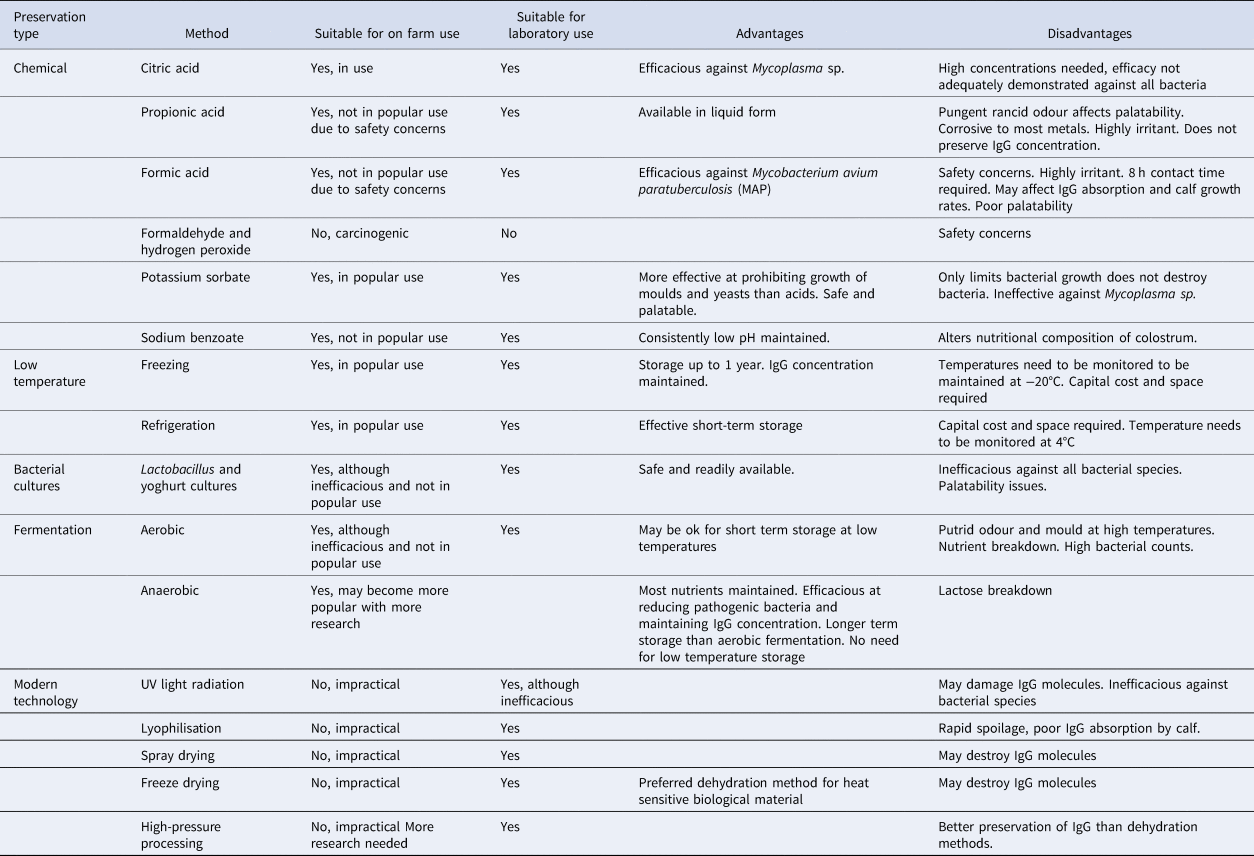
Table 1 summarises each of the preservation options available. Limited work has been done on chemical acidification of colostrum, but work on milk replacer and milk would suggest that palatability and digestibility issues may prohibit its use. IgG absorption from acidified colostrum may also be impaired. Lactobacillus cultures added to colostrum are inefficacious. Controlled anaerobic fermentation of colostrum may provide an alternative to low temperature storage facilities where these are unavailable, whereas potassium sorbate additives could be useful where colostrum is left at ambient temperatures for more than 6 h before feeding to newborn calves. Heat treatment of colostrum is useful to control pathogenic bacteria and reduce overall bacteria counts, but pasteurisation units are costly.
Table 1. Summary table of options for preservation of bovine colostrum detailing suitability for on-farm and laboratory use and advantages and disadvantages of each method
Opportunities for further research
Little recent work has been published on alternative chemical preservatives or explored new technologies to preserve bovine colostrum on farm. Currently, the most promising avenues for future work include exploring user friendly on-farm technology for high pressure processing as this preserves IgG molecules more effectively than UV light and dehydration methods. There is also plenty of scope for more research into practical, on farm colostrum preservation techniques which preclude the requirement for large low temperature storage devices (such as refrigerators and freezers) and allow colostrum to be stored at room temperature. With more local focused research, industry bodies, veterinarians and other agricultural professionals could collaborate to create a ‘joined up’ approach to extension messaging of use of preservatives such as potassium sorbate to best effect. In addition, extension messaging of local research on anaerobic fermentation, including how to optimise and practically perform this type of preservation are currently lacking. Seasonal, tropical and low income production systems would most benefit from employing this type of preservation where colostrum is produced in abundance or low temperature storage options are in short supply.
Acknowledgements
This work was supported by MSD Animal Health Ltd. Thank you to Prof. Nick Jonsson for his support and encouragement in submission of the manuscript for publication.
Conflict of interest
Funding was provided by MSD Animal Health Ltd, however the funders were not involved in the preparation of this manuscript for publication. The author does not have any other financial or personal relationships that could inappropriately influence or bias the content of the paper.



