Milk is the sole and primary source of nutrition for newborns, and it has been demonstrated to be important for children's growth and adult health (Zhang et al., Reference Zhang, Chen, Xu, Yang, Du, Li and Zhou2021). The beneficial effects are related to the presence of different nutrients, including protein, fat, lactose, essential minerals including calcium and magnesium, fat-soluble vitamins (A, D, E, and K) and bioactive compounds (Gil and Ortega, Reference Gil and Ortega2019; Scholz-Ahrens et al., Reference Scholz-Ahrens, Ahrens and Barth2020). Among these bioactive compounds are polyphenols, peptides and polyunsaturated fatty acids (PUFA), which are related to improved health. For children and adults, the main sources of milk and dairy products are dairy cattle, buffaloes, goats and sheep (Ferro et al., Reference Ferro, Tedeschi and Atzori2017). However, the chemical composition and presence of different bioactive compounds in the milk of these species can fluctuate due to several factors, such as animal breed, stage of lactation, season, management and nutrition. Many strategies have been implemented to enhance dairy ruminant product quality, one of which is to focus on the influence of dietary supplementation.
The enrichment of ruminant diets with agro-industrial by-products (for example, citrus pulp, grape pomace and pulp, molasses and olive leaves) that are rich in bioactive compounds such as polyphenols has been demonstrated to improve the nutritional and chemical composition of milk and dairy products (Křížová et al., Reference Křížová, Křešťáková, Dadáková and Kašparovský2021). Dietary supplementation has an important role regarding the presence of antioxidants in milk and dairy products. For example, supplementation with citrus pulp (9–18%) increased the polyphenol and flavonoid concentrations in Holstein milk (Santos et al., Reference Santos, Lima, Schogor, Romero, De Marchi, Grande, Santos, Santos and Kazama2014). The identification of these types of compounds is important because they are thought to have health benefits, such as lowering blood pressure, stopping Gram-negative pathogens like Escherichia coli and Salmonella typhi (Murakami et al., 2004) and having anti-inflammatory and antioxidant effects (Marcone et al., Reference Marcone, Belton and Fitzgerald2017).
Fatty acids also play a significant role in the nutritional value of ruminant products and can be influenced by the enrichment of ruminant diets. The type and quantity of fatty acids in milk are influenced by the animal's breed, lactation stage, husbandry and diet (Tzamaloukas et al., Reference Tzamaloukas, Neofytou, Simitzis and Miltiadou2021). Ianni et al., Reference Ianni, Innosa, Martino, Bennato and Martino2019 found that adding grape pomace to the diet of Friesian cows increased the concentration of polyphenols and linoleic (C18:3n-3), vaccenic (C18:1 trans 11) and rumenic (C18:2 cis 9, trans 11) acids in the cheese. It is known that polyunsaturated fatty acid (PUFA) content in milk plays an important role in consumer health (Chilliard and Ferlay, Reference Chilliard and Ferlay2004). Thus, this review aimed to summarize evidence of the effects of dietary supplementation with antioxidants and phenolic compounds on the milk and dairy products of the main dairy animals.
Materials and methods
The present study was performed following the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines (Page et al., Reference Page, McKenzie, Bossuyt, Boutron, Hoffmann, Mulrow, Shamseer, Tetzlaff, Akl and Brennan2021). The study design required neither Institutional Review Board approval nor patient informed consent.
Search strategy
Two authors (AAN and IMV) performed the search strategy independently. The studies were identified through the online source MEDLINE/PubMed. The search was conducted for articles published until July 2022. We applied the description of the population, intervention, control and outcomes (PICO) strategy as described by Methley et al. (Reference Methley, Campbell, Chew-Graham, McNally and Cheraghi-Sohi2014), where the population was dairy ruminants, the interventions were bioactive compounds and antioxidants in diets, a standard ruminant diet was the control and the outcomes were the fatty acid profile, bioactive compounds and antioxidant levels in the milk and dairy products of the population (online Supplementary Table S1).
Potential articles were searched using keywords by constructing blocks of descriptors in English. The Boolean operators AND (to add at least one word from each group) and OR (to list at least one word from each block), parentheses (to combine search terms by outcome categories) and quotation marks (to search for exact terms or expressions) were used. The groups of descriptors for the search strategy related to the outcome in dairy products were Ruminants AND bioactive compound AND dairy products NOT a review, Ruminants AND bioactive compound supplementation AND dairy product.
Selection of studies
After removing duplicates, the same authors (AAN and IMV) independently screened the titles and abstracts for eligibility evaluation based on the inclusion criteria. The title and abstract candidates to enter the review were evaluated in accordance with their eligibility criteria by all authors. Finally, data extraction of the full texts was carried out. Original studies were included if they met the following criteria:
(1) performed on ruminants (for this review, the search only focused on dairy goats, cows and sheep)
(2) reporting dietary interventions that included bioactive compounds and antioxidants in the diet of ruminants
(3) a design with a standard diet as a comparator
(4) reporting change or concentration of the fatty acid profile, bioactive compounds and antioxidants in milk and dairy products of the ruminants.
Exclusion criteria were:
(1) in vitro studies
(2) characterization of antioxidant concentrations in milk and dairy products without intervention studies
(3) studies where the intervention was focused on comparing feeding by different types of grazing and did not supplement.
Data extraction
Data extraction for all selected articles was performed independently by all authors. This information included the ruminant species, the bioactive compound(s), the description of the intervention, the comparator, the follow-up time, the type of product studied, the main findings about dairy products and finally the first author's name and year of publication. The process of identification and extraction is given at online Supplementary Fig. S1 and the complete list of references is at online Supplementary Table S2.
Results
According to the search, 94 articles were identified, and when duplicates were excluded 81 records were evaluated with title and abstract. In accordance with the eligibility criteria 66 articles were excluded (online Supplementary Table S2). The principal reasons to exclude articles by title and abstract were: no-intervention (n = 17), no-intervention in ruminants (n = 14), food characterization study (n = 12), in vitro study (n = 8), intervention different from the stated objective (n = 6), study of supplementation food or food creation (n = 5), review study (n = 2) and interventions that did not involve bioactive compounds (n = 2). Finally, 15 articles were included in the review (online Supplementary Fig. S1).
Animal models, study designs and dairy products
The studies included in the analysis showed a range of publications from 2014 to 2022 (Table 1). Animal models included were sheep (2 papers), cows (9 papers) and goats (4 papers). Some studies also reported the progeny of the animals such as primiparous (Safari et al., Reference Safari, Ghasemi, Alikhani and Ansari-Mahyari2018; Li et al., Reference Li, Lei, Chen, Yin, Shen and Yao2021) and multiparous (Hausmann et al., Reference Hausmann, Deiner, Patra, Immig, Starke and Aschenbach2018; Safari et al., Reference Safari, Ghasemi, Alikhani and Ansari-Mahyari2018; Li et al., Reference Li, Lei, Chen, Yin, Shen and Yao2021; Wang et al., Reference Wang, Sun, Tu, Si, Liu, Yang, Luo and Yu2021) and included the period of lactation (Ianni et al., Reference Ianni, Innosa, Martino, Bennato and Martino2019, Reference Ianni, Innosa, Oliva, Bennato, Grotta, Saletti, Pomilio, Sergi and Martino2021; Bonanno et al., Reference Bonanno, Di Grigoli, Todaro, Alabiso, Vitale, Di Trana, Giorgio, Settanni, Gaglio and Laddomada2019b; Mapato et al., Reference Mapato, Viennasay, Cherdthong and Wanapat2021; Menci et al., Reference Menci, Natalello, Luciano, Priolo, Valenti, Difalco, Rapisarda, Caccamo, Constant and Niderkorn2021) or specified mid-lactation (Scuderi et al., Reference Scuderi, Ebenstein, Lam, Kraft and Greenwood2019; Simitzis et al., Reference Simitzis, Massouras, Goliomytis, Charismiadou, Moschou, Economou, Papadedes, Lepesioti and Deligeorgis2019). Design of the studies included randomized (Cais-Sokolińska et al., Reference Cais-Sokolińska, Pikul, Wójtowski, Danków, Teichert, Czyżak-Runowska and Bagnicka2015; Hausmann et al., Reference Hausmann, Deiner, Patra, Immig, Starke and Aschenbach2018; Safari et al., Reference Safari, Ghasemi, Alikhani and Ansari-Mahyari2018; Ianni et al., Reference Ianni, Innosa, Martino, Bennato and Martino2019; Bonanno et al., Reference Bonanno, Di Grigoli, Vitale, Di Miceli, Todaro, Alabiso, Gargano, Venturella, Anike and Isikhuemhen2019a; Walkenhorst et al., Reference Walkenhorst, Leiber, Maeschli, Kapp, Spengler-Neff, Faleschini, Garo, Hamburger, Potterat and Mayer2020; Li et al., Reference Li, Lei, Chen, Yin, Shen and Yao2021; Wang et al., Reference Wang, Sun, Tu, Si, Liu, Yang, Luo and Yu2021), stratified (Scuderi et al., Reference Scuderi, Ebenstein, Lam, Kraft and Greenwood2019; Walkenhorst et al., Reference Walkenhorst, Leiber, Maeschli, Kapp, Spengler-Neff, Faleschini, Garo, Hamburger, Potterat and Mayer2020), allocated (Delgadillo-Puga et al., Reference Delgadillo-Puga, Cuchillo-Hilario, León-Ortiz, Ramírez-Rodríguez, Cabiddu, Navarro-Ocaña, Morales-Romero, Medina-Campos and Pedraza-Chaverri2019; Simitzis et al., Reference Simitzis, Massouras, Goliomytis, Charismiadou, Moschou, Economou, Papadedes, Lepesioti and Deligeorgis2019) and 4 × 4 Latin square design with a 2 × 2 factorial arrangement (Mapato et al., Reference Mapato, Viennasay, Cherdthong and Wanapat2021). Dairy products evaluated in different studies included kefir (Cais-Sokolińska et al., Reference Cais-Sokolińska, Pikul, Wójtowski, Danków, Teichert, Czyżak-Runowska and Bagnicka2015), milk (all other papers) and, additionally, cheese (Ianni et al., Reference Ianni, Innosa, Martino, Bennato and Martino2019; Bonanno et al., Reference Bonanno, Di Grigoli, Vitale, Di Miceli, Todaro, Alabiso, Gargano, Venturella, Anike and Isikhuemhen2019a, Reference Bonanno, Di Grigoli, Todaro, Alabiso, Vitale, Di Trana, Giorgio, Settanni, Gaglio and Laddomada2019b; Menci et al., Reference Menci, Natalello, Luciano, Priolo, Valenti, Difalco, Rapisarda, Caccamo, Constant and Niderkorn2021).
Table 1. Bioactive compounds and nutritional interventions used in dairy cattle and its effect on milk and dairy products
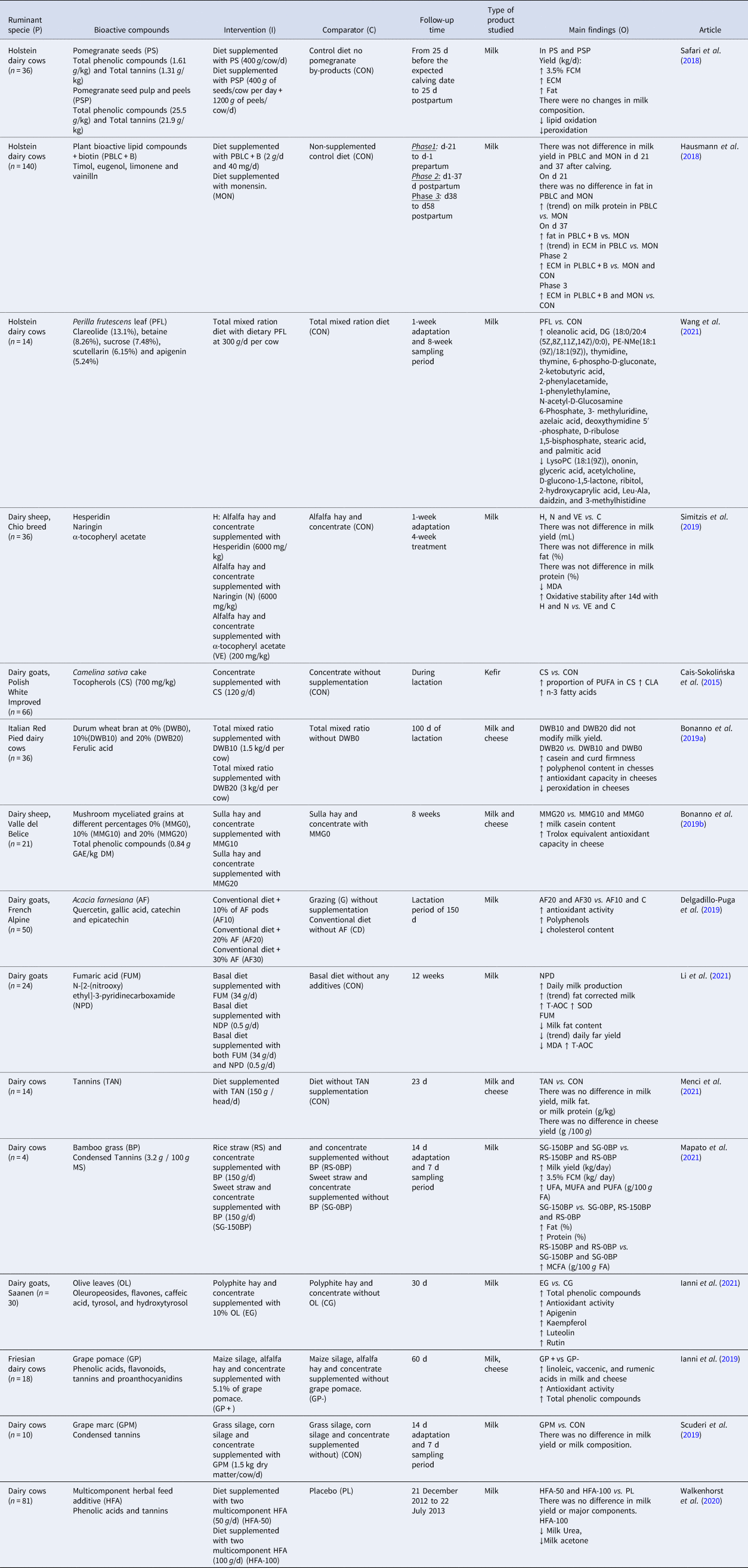
Abbreviations: d, days; FCM, Fat-corrected milk; ECM, Energy-corrected milk; PUFA, polyunsaturated fatty acids; CLA, conjugated linoleic acid; CON, Control.
Types of supplementation
Interventions with plants, herbs, seeds, grains and isolated bioactive compounds were used in the studies. Among the plants included in the interventions were Acacia farnesiana (Delgadillo-Puga et al., Reference Delgadillo-Puga, Cuchillo-Hilario, León-Ortiz, Ramírez-Rodríguez, Cabiddu, Navarro-Ocaña, Morales-Romero, Medina-Campos and Pedraza-Chaverri2019), bamboo grass (Mapato et al., Reference Mapato, Viennasay, Cherdthong and Wanapat2021), olive leaves (Ianni et al., Reference Ianni, Innosa, Oliva, Bennato, Grotta, Saletti, Pomilio, Sergi and Martino2021), herbal feed additives (Walkenhorst et al., Reference Walkenhorst, Leiber, Maeschli, Kapp, Spengler-Neff, Faleschini, Garo, Hamburger, Potterat and Mayer2020) and Perilla frutescens leaf (Wang et al., Reference Wang, Sun, Tu, Si, Liu, Yang, Luo and Yu2021). Other studies included seeds such as false flax cake (Cais-Sokolińska et al., Reference Cais-Sokolińska, Pikul, Wójtowski, Danków, Teichert, Czyżak-Runowska and Bagnicka2015) and pomegranate seeds (Safari et al., Reference Safari, Ghasemi, Alikhani and Ansari-Mahyari2018). There were studies whose interventions were based on food components like durum wheat bran (Bonanno et al., Reference Bonanno, Di Grigoli, Todaro, Alabiso, Vitale, Di Trana, Giorgio, Settanni, Gaglio and Laddomada2019b), grape pomace or marc (Ianni et al., Reference Ianni, Innosa, Martino, Bennato and Martino2019; Scuderi et al., Reference Scuderi, Ebenstein, Lam, Kraft and Greenwood2019) and mushroom myceliated grains (Bonanno et al., Reference Bonanno, Di Grigoli, Vitale, Di Miceli, Todaro, Alabiso, Gargano, Venturella, Anike and Isikhuemhen2019a). Intervention with isolated bioactive compounds included plant bioactive lipid compounds plus biotin and monensin (Hausmann et al., Reference Hausmann, Deiner, Patra, Immig, Starke and Aschenbach2018), hesperidin or naringin or α-tocopheryl acetate (Simitzis et al., Reference Simitzis, Massouras, Goliomytis, Charismiadou, Moschou, Economou, Papadedes, Lepesioti and Deligeorgis2019), tannin extract (Menci et al., Reference Menci, Natalello, Luciano, Priolo, Valenti, Difalco, Rapisarda, Caccamo, Constant and Niderkorn2021), and finally N-[2-(nitrooxy) ethyl]-3-pyridinecarboxamide combined with fumaric acid (Li et al., Reference Li, Lei, Chen, Yin, Shen and Yao2021). The main beneficial effects of the dairy interventions are summarized in Table 1.
Effects of supplementation on fatty acids profile of dairy products
Six studies investigated the impact of dietary intervention on the fat content of milk. Supplementation with plant bioactive lipid compounds and biotin (PBLC + B: Hausmann et al., Reference Hausmann, Deiner, Patra, Immig, Starke and Aschenbach2018), fumaric acid (FUM: Li et al., Reference Li, Lei, Chen, Yin, Shen and Yao2021), rice straw and sweet grass (Mapato et al., Reference Mapato, Viennasay, Cherdthong and Wanapat2021) all promoted an increase in milk fat concentration. It has been reported that cattle diets have an important role in properties such as milk fat composition (Chen et al., Reference Chen, Grandison and Lewis2017), particularly grass feeding and grazing, which promote higher levels of PUFA in comparison with concentrate (Mohan et al., Reference Mohan, O'Callaghan, Kelly and Hogan2021).
Due to its high phytochemical content, pomegranate has been widely studied for its health properties, and several products aimed at human health have been developed, causing an increase in agro-industrial residues (Varma et al., Reference Varma, Shabtay, Yishay, Mizrahi, Shterzer, Freilich, Medina, Agmon and Laor2018). Pomegranate peel extract and pomegranate pulp improve in vitro dry matter digestibility and volatile fatty acid production (Jami et al., Reference Jami, Shabtay, Nikbachat, Yosef, Miron and Mizrahi2012; Shaani et al., Reference Shaani, Eliyahu, Mizrahi, Yosef, Ben-Meir, Nikbachat, Solomon, Mabjeesh and Miron2016). Thus, the use of pomegranate residues in ruminant feed might have an important role in milk production. However, interventions with pomegranate seeds or pomegranate seed pulp did not show differences in milk fat (Safari et al., Reference Safari, Ghasemi, Alikhani and Ansari-Mahyari2018).
There were seven instances of lipid profile being altered by supplementation. False flax cake increased the content of PUFA (by 1.5 times) and n-3 fatty acid levels (by 1.7 times) compared to the control group (Cais-Sokolińska et al., Reference Cais-Sokolińska, Pikul, Wójtowski, Danków, Teichert, Czyżak-Runowska and Bagnicka2015). Intervention with sweet grass increased the concentration of monounsaturated fatty acids (MUFA) and PUFA by modest but significant amounts compared with rice straw (Mapato et al., Reference Mapato, Viennasay, Cherdthong and Wanapat2021). It is important to increase the content of PUFA in dairy products because it has been established that PUFA, among other health benefits, regulates the inflammatory response (Bentsen, Reference Bentsen2017). During this process, immune cells produce inflammatory mediators such as tumor necrosis factor-alpha, interleukin (IL)-1beta, IL-6, IL-12, interferon gamma, and IL-8. These mediators activate pro-inflammatory signaling cascades, including the nuclear factor-kB (NF-kB) signaling pathway, the Janus kinase/signal transducer and activator of transcription signaling pathway and the mitogen-activated protein kinase signaling pathway (Kahkhaie et al., Reference Kahkhaie, Mirhosseini, Aliabadi, Mohammadi, Mousavi, Haftcheshmeh, Sathyapalan and Sahebkar2019). It has been demonstrated that PUFA, specifically n-3 PUFA, inhibits the synthesis of IL-1, IL-2, and IL-6 and the NF-kB signaling pathway (Oppedisano et al., Reference Oppedisano, Macrì, Gliozzi, Musolino, Carresi, Maiuolo, Bosco, Nucera, Caterina Zito and Guarnieri2020). Thus, the increase in PUFA concentration observed in the evaluated supplementations indicates that it is possible to enhance the nutritional value of milk and, therefore, might improve the consumer health. It must be cautioned that there is no direct evidence for this, nevertheless, it is an exciting prospect.
Among the PUFA compounds that showed the most change through interventions were linolenic acid, linoleic acid, eicosapentaenoic acid (EPA), and docosahexaenoic acid (DHA). Intervention with Acacia farnesiana at 30% significantly increased the concentration in milk of linoleic acid and DHA compared with control (Delgadillo-Puga et al., Reference Delgadillo-Puga, Cuchillo-Hilario, León-Ortiz, Ramírez-Rodríguez, Cabiddu, Navarro-Ocaña, Morales-Romero, Medina-Campos and Pedraza-Chaverri2019). Similarly, dietary supplementation with dried grape pomace promoted an increase in the percentage of linoleic acid and linolenic acid (Ianni et al., Reference Ianni, Innosa, Martino, Bennato and Martino2019). One of these studies showed that EPA was only detected in the milk of groups fed mushroom myceliated grains (MMG), but this intervention did not show an effect on other PUFA such as linoleic (C18:2 n-6), rumenic (CLA, C18:2 c9 t11), α-linolenic (C18:3 n-3) and arachidonic (C20:4 n-6) acids (Bonanno et al., Reference Bonanno, Di Grigoli, Todaro, Alabiso, Vitale, Di Trana, Giorgio, Settanni, Gaglio and Laddomada2019b). Also, sweet grass increased the proportion of C18:1 cis-9, C18:2, C18:2 cis-9, trans-11 and C18:3 (Mapato et al., Reference Mapato, Viennasay, Cherdthong and Wanapat2021). PUFA have an important role as active dietary compounds, particularly CLA has shown different beneficial effects, such as antihypertensive and anti-carcinogenic activities (Koba and Yanagita, Reference Koba and Yanagita2014). In this aspect, when compared to women who consumed 1 serving/d, those who consumed >4 servings of high-fat dairy foods and CLA per day (including whole milk, full-fat cultured milk, cheese, cream, sour cream and butter) had a tendency to decrease the incidence of colorectal cancer (rate ratio = 0.59 [95% CI: 0.44, 0.79; P for trend = 0.002]), and the increment of 2 servings of high-fat dairy foods/d decreased by 13% risk of colorectal cancer (multivariate rate ratio: 0.87, 95% CI: 0.78, 0.96). Thus, consuming high-fat dairy products rich in CLA may lower the risk of developing colorectal cancer (Larsson et al., Reference Larsson, Bergkvist and Wolk2005). Once again, a caveat is needed since these effects are small and have not been confirmed in bigger studies.
A further note of caution is needed. Supplementation not only showed an effect on these potentially beneficial fatty acids but also promoted an effect on saturated fatty acids. Supplementation with MMG increased the amounts of saturated fatty acids (80.3 vs. 77.94g/100 g of fatty acid, P < 0.01: Bonanno et al., Reference Bonanno, Di Grigoli, Vitale, Di Miceli, Todaro, Alabiso, Gargano, Venturella, Anike and Isikhuemhen2019a). For several years, the consumption of saturated fatty acids was associated with the prevalence of cardiovascular disease (Siri-Tarino et al., Reference Siri-Tarino, Sun, Hu and Krauss2010) and metabolic diseases such as metabolic syndrome and type 2 diabetes (Warensjö et al., Reference Warensjö, Risérus and Vessby2005), however, recent evidence indicates that there is a clear difference between dietary and circulating saturated fatty acids, and multiple studies indicate that there is no association between the consumption of saturated fatty acids and the risk of chronic disease (Astrup et al., Reference Astrup, Magkos, Bier, Brenna, de Oliveira Otto, Hill, King, Mente, Ordovas and Volek2020). It is important to note that milk and dairy products are food matrix foods rich in saturated fatty acids and beneficial compounds such as PUFA, and their consumption should not be associated with an increase in cardiovascular and metabolic risk.
Four studies showed the effect of the intervention on the fat composition of cheese. Supplementation with grape pomace resulted in a significant increase in the concentration of oleic acid, linoleic acid and rumenic acid (all P < 0.01) compared with the control group (Ianni et al., Reference Ianni, Innosa, Martino, Bennato and Martino2019) and intervention with tannin extract during the dry season increased the concentration of conjugated linoleic acid (Menci et al., Reference Menci, Natalello, Luciano, Priolo, Valenti, Difalco, Rapisarda, Caccamo, Constant and Niderkorn2021). However, durum wheat bran and MGG supplementation did not modify the chemical composition of the cheeses (Bonanno et al., Reference Bonanno, Di Grigoli, Vitale, Di Miceli, Todaro, Alabiso, Gargano, Venturella, Anike and Isikhuemhen2019a, Reference Bonanno, Di Grigoli, Todaro, Alabiso, Vitale, Di Trana, Giorgio, Settanni, Gaglio and Laddomada2019b) despite the latter's effect on milk composition.
Identification of bioactive compounds in dairy products altered by supplementation
One of the most important aspects of interventions with bioactive compounds in the feeding of dairy animals is that these compounds need to be bioavailable in the products obtained. Among the studies included, five reported the presence of bioactive compounds in milk (Delgadillo-Puga et al., Reference Delgadillo-Puga, Cuchillo-Hilario, León-Ortiz, Ramírez-Rodríguez, Cabiddu, Navarro-Ocaña, Morales-Romero, Medina-Campos and Pedraza-Chaverri2019; Ianni et al., Reference Ianni, Innosa, Martino, Bennato and Martino2019, Reference Ianni, Innosa, Oliva, Bennato, Grotta, Saletti, Pomilio, Sergi and Martino2021; Bonanno et al., Reference Bonanno, Di Grigoli, Vitale, Di Miceli, Todaro, Alabiso, Gargano, Venturella, Anike and Isikhuemhen2019a, Reference Bonanno, Di Grigoli, Todaro, Alabiso, Vitale, Di Trana, Giorgio, Settanni, Gaglio and Laddomada2019b) and three in the composition of cheese (Ianni et al., Reference Ianni, Innosa, Martino, Bennato and Martino2019; Bonanno et al., Reference Bonanno, Di Grigoli, Vitale, Di Miceli, Todaro, Alabiso, Gargano, Venturella, Anike and Isikhuemhen2019a, Reference Bonanno, Di Grigoli, Todaro, Alabiso, Vitale, Di Trana, Giorgio, Settanni, Gaglio and Laddomada2019b). The inclusion of Acacia farnesiana at 20 and 30% in the diet of goats significantly increased the total phenolic content in the milk and bioactive compounds such as gallic, chlorogenic, ferulic acids and catechin were only detected in milk from supplemented goats (Delgadillo-Puga et al., Reference Delgadillo-Puga, Cuchillo-Hilario, León-Ortiz, Ramírez-Rodríguez, Cabiddu, Navarro-Ocaña, Morales-Romero, Medina-Campos and Pedraza-Chaverri2019). The same group tested the impact of consuming goat milk supplemented with 30% Acacia farnesiana in conjunction with a high-fat diet to assess metabolic alterations in a mouse model, and decreased body weight and body fat mass, improved glucose tolerance, and prevention of hypertrophy of adipose tissue and hepatic steatosis. The effect of supplementation on body weight and body fat mass could be explained because a higher energy expenditure was documented, evidenced by a higher oxygen consumption in indirect calorimetry. Additionally, it has been documented that a lower amount of lipids in brown adipose tissue is related to an increased abundance of uncoupling protein 1. The effects demonstrated in this study might indicate that the consumption of goat's milk supplemented with Acacia farnesiana would be a dietary strategy to improve the metabolic alterations induced by the high-fat diet. However, human studies are required before any definitive conclusions can be drawn. According to the body surface area normalization method (FDA, 2005), the mouse dosage would equate to an equivalent daily human intake of 1.4–2.8 cups (250 ml per cup/d) of fresh goat's milk for a 60 kg adult, so this dose could be the reference to show its effectiveness in clinical studies.
Supplementation with grape pomace caused an increase in the total phenolic compounds in milk in comparison with the non-supplemented group (Ianni et al., Reference Ianni, Innosa, Martino, Bennato and Martino2019). Grape pomace is a product with prebiotic activity because it contains up to 75% dietary fiber (Yu and Ahmedna, Reference Yu and Ahmedna2013), however, the prebiotic effect in addition to the fiber could be given by the phenolic compounds, as they also have a significant effect on the composition and activity of the intestinal microbiota by stimulating or inhibiting specific bacterial groups (Seo et al., Reference Seo, Kim, Jeong, Yokoyama and Kim2017). Phenolic compounds are poorly absorbed in the small intestine and do, therefore, reach the colon, where they are metabolized by the resident microbiota into biologically active metabolites (Ozdal et al., Reference Ozdal, Sela, Xiao, Boyacioglu, Chen and Capanoglu2016). This results in the appearance of a wide range of phenolic metabolites (phenylacetic acids, phenylpropionic acids, valeric acids, cinnamic acids, benzoic acids, and phenols, among others: Mena et al., Reference Mena, Bresciani, Brindani, Ludwig, Pereira-Caro, Angelino, Llorach, Calani, Brighenti and Clifford2019). Grape derivatives, such as phenolic compounds, can promote the growth of probiotic bacteria, including Bifidobacterium teenageris, Bifidobacterium bifidum, Lactobacillus acidophilus, and Lactobacillus rhamnosus (Parkar et al., Reference Parkar, Stevenson and Skinner2008; Gwiazdowska et al., Reference Gwiazdowska, Juś, Jasnowska-Małecka and Kluczyńska2015). This modulation in the intestinal microbiota has a positive effect on the health of the host because experimental studies have shown effects in decreasing weight, waist circumference and fat mass and also in decreasing insulin resistance after probiotic treatments, mainly Lactobacillus and Bifidobacterium (Ejtahed et al., Reference Ejtahed, Angoorani, Soroush, Atlasi, Hasani-Ranjbar, Mortazavian and Larijani2019).
Unfortunately, phenolic compounds are not particularly stable in refrigerated milk. Dairy products that were enriched with polyphenolic compounds showed a decrease in the phenolic content after 28 d of refrigerated storage, which was attributed to their oxidation (Deolindo et al., Reference Deolindo, Monteiro, Santos, Cruz, da Silva and Granato2019). One option to prevent oxidation would be the encapsulation of polyphenols, since encapsulation can protect the bioactive compounds from oxidation. However, encapsulation is more favorable for the enrichment of the dairy product than for the interventions to the diets of the ruminants.
Olive leaf supplementation is another intervention that significantly increases the concentration of phenolic compounds in milk. The main compounds detected in this milk were cinnamic acid, chlorogenic acid and tyrosol (Ianni et al., Reference Ianni, Innosa, Oliva, Bennato, Grotta, Saletti, Pomilio, Sergi and Martino2021). An interesting finding in the profile of bioactive compounds in milk was the content of chlorogenic acids (CGAs), since these are among the most common bioactive compounds in plant foods such as coffee, apples, tea and berries, as well as in beverages such as wine (Zanotti et al., Reference Zanotti, Dall'Asta, Mena, Mele, Bruni, Ray and Del Rio2015). These compounds are esters that are made when quinic acid and trans-cinnamic acids join together. They are usually partially absorbed in the small intestine and partially absorbed in the large intestine after being broken down by bacteria (Olthof et al., Reference Olthof, Hollman and Katan2001, Reference Olthof, Hollman, Buijsman, Van Amelsvoort and Katan2003). The concentration of CGAs in milk is relevant because, according to the literature, their consumption could have an important impact on the improvement of glucose and lipid metabolism. Various mechanisms have been proposed, including that they are involved in the inhibition of α-amylase, an enzyme responsible for the decomposition of starch present in saliva that inhibits the absorption of sugar from diet (Narita and Inouye, Reference Narita and Inouye2009). In addition, they could modulate gastrointestinal peptides such as gastric inhibitory polypeptide and glucagon-like peptide 1 (Johnston et al., Reference Johnston, Clifford and Morgan2003) as well as stimulating glucose transporter 4, thereby increasing glucose uptake by peripheral tissues (Song et al., Reference Song, Choi and Park2014). All these mechanisms result in a significant reduction in blood glucose levels (Van Dam, Reference Van Dam2006). On the other hand, for lipid metabolism it has been shown that CGAs could down-regulate sterol regulatory element-binding protein 1C (Murase et al., Reference Murase, Misawa, Minegishi, Aoki, Ominami, Suzuki, Shibuya and Hase2011) which is the main genetic switch that controls lipogenesis. Both CGA and caffeic acid stimulate the peroxisome expression of nuclear transcription receptor proliferator-activated receptor alpha in obese mice induced by a high-fat diet. This receptor, when activated, acts as a sensor of lipids, and regulates lipid metabolism. The liver is its main target tissue, and its key genes are enzymes involved in the β-oxidation of fatty acids (Cho et al., Reference Cho, Jeon, Kim, Yeo, Seo, Choi and Lee2010). Although highly speculative, all of this together may result in improvements in lipid metabolism. However, not all supplementations have positive effects. Intervention with durum wheat bran (20%) only showed a tendency in phenolic compounds in milk with respect to the control group (Bonanno et al., Reference Bonanno, Di Grigoli, Todaro, Alabiso, Vitale, Di Trana, Giorgio, Settanni, Gaglio and Laddomada2019b), and none of the cheese studies yielded positive changes on total phenolic compounds.
Antioxidant compounds in dairy products affected by supplementation
An outcome of interest from supplementation is the antioxidant effect generated by dairy products. Of the fifteen selected studies, six of these reported an antioxidant effect in milk after the intervention of Acacia farnesiana (Delgadillo-Puga et al., Reference Delgadillo-Puga, Cuchillo-Hilario, León-Ortiz, Ramírez-Rodríguez, Cabiddu, Navarro-Ocaña, Morales-Romero, Medina-Campos and Pedraza-Chaverri2019), hesperidin or naringin (Simitzis et al., Reference Simitzis, Massouras, Goliomytis, Charismiadou, Moschou, Economou, Papadedes, Lepesioti and Deligeorgis2019), durum wheat bran (Bonanno et al., Reference Bonanno, Di Grigoli, Todaro, Alabiso, Vitale, Di Trana, Giorgio, Settanni, Gaglio and Laddomada2019b), MMG (Bonanno et al., Reference Bonanno, Di Grigoli, Vitale, Di Miceli, Todaro, Alabiso, Gargano, Venturella, Anike and Isikhuemhen2019a), grape pomace (Ianni et al., Reference Ianni, Innosa, Martino, Bennato and Martino2019), and olive leaves (Ianni et al., Reference Ianni, Innosa, Oliva, Bennato, Grotta, Saletti, Pomilio, Sergi and Martino2021). Intervention with Acacia farnesiana at different concentrations significantly increased antioxidant activity determined both by oxygen radical absorbance capacity assay and ferric reducing antioxidant power assay compared with conventional diet (Delgadillo-Puga et al., Reference Delgadillo-Puga, Cuchillo-Hilario, León-Ortiz, Ramírez-Rodríguez, Cabiddu, Navarro-Ocaña, Morales-Romero, Medina-Campos and Pedraza-Chaverri2019). Similarly, supplementation with MMG at 20% showed a significant increase in total equivalent antioxidant capacity with respect to the control without interventions (Bonanno et al., Reference Bonanno, Di Grigoli, Vitale, Di Miceli, Todaro, Alabiso, Gargano, Venturella, Anike and Isikhuemhen2019a), antioxidant activity in milk from animals that were fed grape pomace (Ianni et al., Reference Ianni, Innosa, Martino, Bennato and Martino2019) or olive leaf (Ianni et al., Reference Ianni, Innosa, Oliva, Bennato, Grotta, Saletti, Pomilio, Sergi and Martino2021) increased significantly compared with the control groups and 14 d of hesperidin, naringin or α-tocopheryl acetate dietary supplementation achieved the same effect (Simitzis et al., Reference Simitzis, Massouras, Goliomytis, Charismiadou, Moschou, Economou, Papadedes, Lepesioti and Deligeorgis2019).
The role of antioxidants is relevant because they can contribute to the reduction of reactive oxygen species (ROS). Where there is an abundance of ROS and a deficiency of antioxidants, oxidative stress is generated, which in turn causes oxidative damage to biomolecules such as DNA, proteins and lipids (Aranda-Rivera et al., Reference Aranda-Rivera, Cruz-Gregorio, Arancibia-Hernández, Hernández-Cruz and Pedraza-Chaverri2022). Since lipid oxidation, also known as lipid peroxidation, produces oxidative biomarkers such as malondialdehyde (MDA) and oxidized LDL (ox-LDL), an increase in these biomarkers has been associated with metabolic alterations and cardiovascular complications (Lee et al., Reference Lee, Margaritis, Channon K and Antoniades2012).
Scientific evidence to support the use of antioxidants from food, such as dairy products, as a strategy to prevent pathologies and/or complications related to oxidative stress would be of considerable value, but simply showing their presence in the raw product is only a part of the solution. There are only a few studies that demonstrate the antioxidant effect of dairy products. One of these studies in a healthy population showed that after 21 d of consumption of goats' milk there was a small but significant increase in the percentage of total antioxidant activity and a decrease in the relationship of levels of the endogenous antioxidant glutathione (oxidized glutathione:reduced glutathione: Kullisaar et al., Reference Kullisaar, Songisepp, Mikelsaar, Zilmer, Vihalemm and Zilmer2003). A second asked participants to consume, for 4 weeks, an experimental cheese that was made from the milk of cows fed a diet containing 5% linseed oil. In this case, serum ox-LDL decreased significantly (Intorre et al., Reference Intorre, Foddai, Azzini, Martin, Montel, Catasta, Toti, Finotti, Palomba and Venneria2011).
It is not only important to consider that antioxidant activity increases, but also that it is able to decrease levels of ROS. Excessive ROS oxidizes cell components, which produce alterations in their structure, causing interruption of signaling pathways or even generating dysfunction of metabolic pathways. Thus, the importance of antioxidants showing this effect on health is to promote benefits in populations with pathologies associated with oxidative stress such as obesity, type 2 diabetes, dyslipidemia and cardiovascular disease (Forrester et al., Reference Forrester, Kikuchi, Hernandes, Xu and Griendling2018). A study that included patients with a diagnosis of metabolic syndrome and a 12-week intervention with several serves of dairy per day (adequate dairy) or less than one (low dairy) showed that adequate dairy consumption significantly decreased levels of MDA and ox-LDL (Stancliffe et al., Reference Stancliffe, Thorpe and Zemel2011). The decrease in these markers is potentially of great importance, however, it is an isolated example and more evidence about the consumption of dairy products in different types of populations is needed to demonstrate the antioxidant effect it may generate.
Discussion
In recent years, bioactive compounds and agro-industrial residues have been used as feed for dairy animals because they may have positive effects on animal production, such as regulating ruminal fermentation, stopping methane production and protein breakdown, boosting the immune response, and increasing antioxidant activities in animal tissues (Niderkorn and Jayanegara, Reference Niderkorn and Jayanegara2021). There are a great variety of bioactive compounds that can be used in the nutrition of dairy animals. In this review, 19 different types of supplements were used. Only one of them, biotin, is an approved additive according to the EU Register on Nutrition Health Claims; pomegranate seed, alfalfa hay, hesperidin, naringin, mushroom myceliated grains, and tannins are not approved. Meanwhile, the remaining interventions do not appear on any list as authorized or non-authorized. To ensure food safety and animal welfare, it is essential to regulate the feeding of dairy animals with bioactive substances and agro-industrial residues.
Milk and milk products are important foods for human nutrition, constituting 25–30% of the diet. Their nutritional value is, in part, associated with the lipid content due to the inclusion of fatty acids, vitamins and minerals (Visioli and Strata, Reference Visioli and Strata2014). Epidemiological data have shown associations between health effects and dairy product intake (Givens, Reference Givens2020). On the other hand, many other studies have linked the consumption of dairy products with the risk of developing pathologies, mainly because of their lipid content (Fontecha and Juárez, Reference Fontecha and Juárez2017). Thus, health policies have suggested the consumption of fat-free milk and milk-derived products to prevent the risk of cardiometabolic pathologies (You, Reference You2015). More recent analysis employing systematic review has shown inconclusive or contradictory results about the health effects of dairy product consumption (Nieman et al., Reference Nieman, Anderson and Cifelli2021). Epidemiological studies have shown an inverse association between the intake of dairy products and hypertension, stroke, and colorectal cancer, but there is no evidence of an association between the consumption of dairy products and breast cancer. There is some weak evidence of the protective capacity of dairy products for bone health (Alvarez-León et al., Reference Alvarez-León, Román-Vinas and Serra-Majem2006). A meta-analysis showed that milk and total dairy products, but not cheese or other dairy products, are associated with a reduction in colorectal cancer risk. Inverse associations were observed in both men and women but were restricted to colon cancer, where there was evidence of a significant nonlinear association between milk and total dairy products and colorectal cancer risk, and the inverse associations appeared to be the strongest at the higher range of intake (Aune et al., Reference Aune, Lau, Chan, Vieira, Greenwood, Kampman and Norat2012). The nutritional benefits of milk and dairy products are undeniable, but the intricacies of specific health or disease consequences are very difficult to establish.
Dairy products may contain antioxidants such as vitamins A and E, which may provide health benefits due to their ability to reduce oxidative stress and inflammation, but note the term ‘may’. Depending on factors such as animal diet, breed and production methods, the antioxidant content of dairy products can vary. Some studies indicate that feeding dairy animal diets rich in antioxidants, such as those containing high levels of vitamin E or plant-based compounds, can increase the antioxidant content of their milk (Delgadillo-Puga et al., Reference Delgadillo-Puga, Cuchillo-Hilario, León-Ortiz, Ramírez-Rodríguez, Cabiddu, Navarro-Ocaña, Morales-Romero, Medina-Campos and Pedraza-Chaverri2019, Reference Delgadillo-Puga, Noriega, Morales-Romero, Nieto-Camacho, Granados-Portillo, Rodríguez-López, Alemán, Furuzawa-Carballeda, Tovar and Cisneros-Zevallos2020), which is encouraging but we are far from understanding whether such increases actually achieve health benefits. Nevertheless, we can say that improving the diet of dairy animals could potentially increase the nutritional value of the milk they produce.
Efforts have been made to maximize the potential health benefits of dairy products and increase their clinical relevance. Studies have demonstrated that improved chemical composition or enrichment with bioactive compounds such as PUFA, peptides and antioxidants can contribute to the enhancement of the quality of these products by promoting positive effects. Recent evidence suggests that benefits from dairy products on health include regulation of carbohydrate and lipid metabolism through effects on abundance and composition of gut microbiota, cardiovascular diseases, type 2 diabetes, modulation of the immune response and decreased risk of different types of cancer (Tong et al., Reference Tong, Dong, Wu, Li and Qin2011; Sharafedtinov et al., Reference Sharafedtinov, Plotnikova, Alexeeva, Sentsova, Songisepp, Stsepetova, Smidt and Mikelsaar2013; Nilsen et al., Reference Nilsen, Høstmark, Haug and Skeie2015; Brassard et al., Reference Brassard, Tessier-Grenier, Allaire, Rajendiran, She, Ramprasath, Gigleux, Talbot, Levy and Tremblay2017; Santurino et al., Reference Santurino, López-Plaza, Fontecha, Calvo, Bermejo, Gómez-Andrés and Gómez-Candela2020).
Changes in these types of products brought about by the presence of bioactive compounds can also have positive outcomes for the production animal. For example, the increase in milk fat concentration due to plant bioactive lipid compounds and biotin can be explained by the prevention of postpartum weight loss and an increase in back fat thickness (Hausmann et al., Reference Hausmann, Deiner, Patra, Immig, Starke and Aschenbach2018). Fumaric acid had a negative impact on total fat content. The authors theorized that this was due to the decreased proportion of precursors for the de novo synthesis of milk fatty acids, such as butyrate and the acetate-to-propionate ratio (Li et al., Reference Li, Lei, Chen, Yin, Shen and Yao2021). The higher production of fat in milk when sweet grass was supplemented was accompanied by an increase in digestibility and feed intake, thereby increasing the nutrients available for the rumen microbes and enhancing rumen fermentation, total milk production and milk composition (Mapato et al., Reference Mapato, Viennasay, Cherdthong and Wanapat2021). Also, sweet grass increases the concentration of MUFA in milk because fresh grass increases milk fatty acids that are ruminal biohydrogenation intermediates (C18:1, C18:2, C18:3). It has been demonstrated that MUFA can improve glycemic control and prevent the development of metabolic syndrome and its complications (Sheashea et al., Reference Sheashea, Xiao and Farag2021). Similar results are shown in the intervention with Acacia farmesiana, dried grape pomace, and MMG that increased the long-chain fatty acids, like linoleic and alpha-linolenic acids. This effect can probably be related to ruminal kinetic modifications due to the rich bioactive compounds found in supplements (Delgadillo-Puga et al., Reference Delgadillo-Puga, Cuchillo-Hilario, León-Ortiz, Ramírez-Rodríguez, Cabiddu, Navarro-Ocaña, Morales-Romero, Medina-Campos and Pedraza-Chaverri2019). Enrichment in the content of PUFA in this study has an important role in consumer health because PUFA are associated with preservation of insulin sensitivity, regulation of blood pressure, adequate coagulation and enhanced endothelial function (Julibert et al., Reference Julibert, del Mar Bibiloni and Tur2019).
The use of tannin extract in the diet, by contrast, had a negative effect on C18:1 trans-10, which may affect the pathway of microbial conversion of C18:3 cis-9, cis-12, and cis-15 to C18:1 trans-10 in the rumen. However, these effects were not reflected on cheese-making parameters (Menci et al., Reference Menci, Natalello, Luciano, Priolo, Valenti, Difalco, Rapisarda, Caccamo, Constant and Niderkorn2021). Mapato et al. (Reference Mapato, Viennasay, Cherdthong and Wanapat2021) found that adding bamboo grass with bioactive compounds like condensed tannins improved the rumen microbiome, which had a positive effect on total volatile fatty acids and propionic acid. The increment of saturated fatty acids due to the inclusion of MMG in the diet was explained by an increment in palmitic acid (C16:0) (Bonanno et al., Reference Bonanno, Di Grigoli, Vitale, Di Miceli, Todaro, Alabiso, Gargano, Venturella, Anike and Isikhuemhen2019a).
Variation in milk composition may also be accompanied by the presence of bioactive compounds such as phenols and ferulic acid in milk, with a subsequent presence in processed products. A positive effect on oxidative damage was observed in cheese, which was less prone to proteolysis during ripening without any changes in sensory characteristics (Ianni et al., Reference Ianni, Innosa, Martino, Bennato and Martino2019). Another example of the antioxidant capacity was also described in cheeses induced by MMG in the diet due to the presence of phenolic acids, flavonoids, polysaccharides, carotenoids, ascorbic-acids, and tocopherols.
In conclusion, consumption of products with antioxidants and an adequate lipid profile can be considered a strategy to prevent the damage caused by oxidative stress (Rani et al., Reference Rani, Deep, Singh, Palle and Yadav2016).
This systematic review compiles scientific studies about supplementation with bioactive compounds to improve the nutrition profile and composition of milk and dairy products. It was observed that supplementation with bioactive compounds in the diet of dairy animals had a positive impact on dairy products, which ranged from an increase in antioxidant capacity to a decrease in metabolites such as malondialdehyde. Future studies should focus on exploring the impact of consuming these products on human health.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0022029923000511



