Introduction
The theory of a Developmental Origin of Health and Disease states that exposure to adverse environmental conditions (e.g. undernutrition, obesity, hypoxia or stress) during development can programme an increased risk of disease in the offspring later in life.Reference Barker 1 – Reference Giussani and Davidge 3 The mechanisms by which this occurs can involve a direct influence of adverse conditions on fetal development or, alternatively, fetal development may also be affected secondary to changes in the maternal and/or placental physiology. Avian species are ideal for isolating the direct effects of adverse conditions on fetal development from any confounding effects via the mother or placenta, as the chicken embryo develops inside the egg completely independent of adverse maternal conditions.
There are several other advantages of using avian species. They have a comparatively short incubation period, meaning studies can be conducted quickly. Compared with rodents, the timing of key events in the development of their nervous and cardiovascular systems is closer to that of human development.Reference Itani, Salinas and Villena 4 , Reference Monie 5 Furthermore, unlike polytocous mammals, there is no need to consider within-litter variation, the maternal investment in the pregnancy or effects of lactation. Hence, there is no need to deal with the complications of using surrogate pregnancies or cross-fostering experimental designs.Reference Matthews, Samuelsson and Seed 6 Drugs can also be easily given into the embryonic circulation by topical administration onto the chorioallantoic membrane.Reference Stewart and Kirby 7 – Reference Tintu, Rouwet and Verlohren 9 Therefore, in addition to isolating the direct programming effects of adverse conditions on the developing individual, it is also possible to isolate the direct effects of potential interventional therapies against programming of disease in the offspring. Finally, there is an additional advantage from an ethical perspective, as there is no need to sacrifice a maternal life in order to study the fetus. Hence, the number of animals required is significantly reduced, thereby abiding strongly by the 3R principle, enshrined in the United Kingdom by the Home Office. However, despite these advantages, surgical techniques, which are invaluable to study in vivo cardiovascular and metabolic function in the adult individual, are not well described for birds. Therefore, the aim of this study was to establish a surgical protocol for in vivo cardiovascular investigation in the adult chicken.
Methods
Animals
All procedures were carried out in accordance with the UK Animals (Scientific Procedures) Act 1986 and were approved by the Local Ethics Review Committee of the University of Cambridge. Surgery was performed on seven female Bovan Brown chickens (Gallus gallus domesticus), aged 6–7 months and weighing 1617±214 g (mean±s.d.). These chickens had just started to lay eggs and so are roughly equivalent to teenage or young adult humans.
Pre-surgical preparation
The chickens were deprived of food but not water 3 h before surgery. Anaesthesia was induced by spontaneous inhalation of 2–2.5% isoflurane (IsoFlo; Abbott Laboratories Ltd, Berkshire, UK) in 4 l/min O2 via a transparent facemask. Once induced, anaesthesia was maintained with 1.5–2% isoflurane and 4–5 LO2/min, administered via the facemask. Chickens were placed in a lateral recumbent position on a heat mat with the head elevated by 5–10 cm to prevent aspiration if any crop reflux occurred during anaesthesia. Throughout surgery, the chickens underwent careful monitoring of their arterial blood oxygen saturation and heart rate (Pulse Oximeter YM-2500; Thames Medical, Sussex, UK) as well as their core temperature (via cloacal thermometer), breathing rate and colour of their comb (visual analysis). The outer thigh region of each leg was exposed by careful plucking of the feathers (Fig. 1a) and the skin gently scrubbed with 70% ethanol in water, followed by Hibitane (Hibitane Plus; Regent Medical Ltd, Manchester, UK) in 70% ethanol and iodine (Povidone-Iodine; Seton Healthcare Group PLC, Oldham, UK).
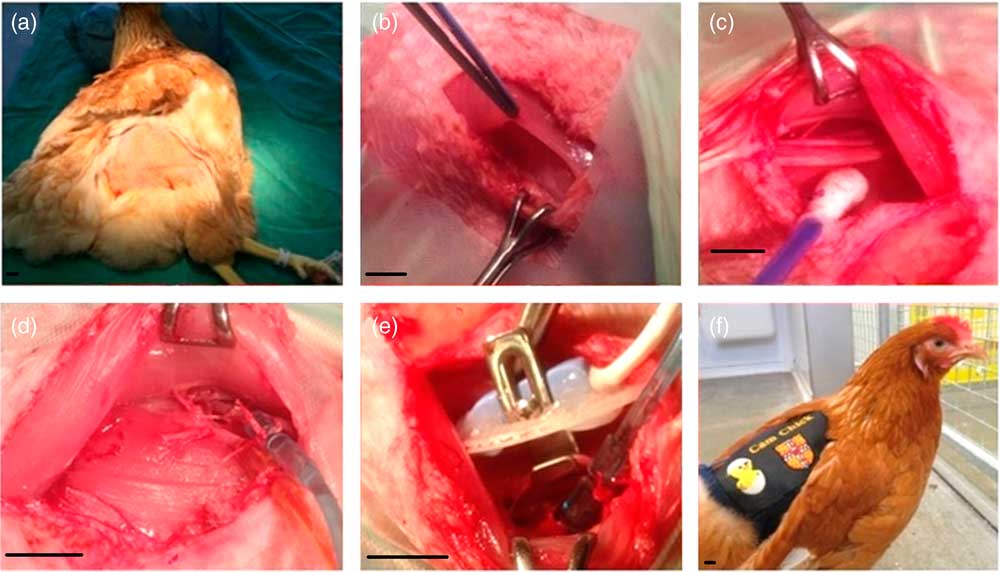
Fig. 1 Chicken surgery. Chickens were anaesthetised, positioned with their head elevated and feathers plucked from each other thigh (a). The iliotibialis and semitendinosus muscles were separated (b), revealing the femoral artery and vein (c). On the first leg both the artery and vein were catheterised (d). On the contralateral leg a Transonic flow probe was placed around the artery (e). Following surgery, the chicken was recovered, and the exteriorised catheters and the flow probe lead housed in a custom-made jacket (f). Scale bars represent 1 cm.
Surgical procedure
All surgical procedures were performed under aseptic conditions. Using a surgical cauteriser, a 2.5–3 cm incision was made superior to the fat pad on the lateral thigh, where vascularity is reduced (Fig. 1b). Locaine (0.2 ml, 1% w/v lidocaine hydrochloride, 0.002% w/v adrenaline acid tartrate; AnimalCare, York, UK) was injected into the iliotibialis muscle using a 25 G needle.
The iliotibialis and semitendinosus muscles were then separated by careful blunt dissection to expose the femoral artery and vein (Fig. 1c). Catheters made from polyvinyl chloride (PVC) tubing (1 mm bore, 0.5 mm wall; Altec, St Austell, UK) with a 10 cm PVC extension catheter glued in place (diameter 0.96 mm, bore 0.58 mm; Critchley Electrical Products Pty Ltd, Silverwater, Australia) and filled with non-heparinised saline (0.9% w/v NaCl; AnimalCare Ltd, York, UK) were inserted into both vessels. In order to catheterise, two ligatures were passed around a 1 cm segment of the vessel, and the distal ligature tied securely to occlude the vessel. Whilst the surgical assistant stopped the flow of blood using the proximal ligature, a small incision was made in the vessel, and the catheter threaded in up to the end of the extension catheter before both ligatures were tied around the catheter to hold it securely in place (Fig. 1d). The catheters were plugged with sterile brass pins (Southerns, Orpington, UK), and, using an exteriorising needle, were tunnelled subcutaneously to the saddle of the chicken to exit through a small incision in the skin. Gelfoam (Pharmacia & Upjohn Co., Michigan, USA) was placed on top of the exposed muscle, and the skin was sewn closed over it using 2.0 Ethilon (Ethicon, San Lorenzo, CA, USA). Once the arterial catheter was in place, it was connected to a pressure transducer (Argon Medical Devices, Plano, TX, USA) filled with heparinised saline (100 i.u./ml heparin in 0.9% NaCl). The transducer was then connected to an M-PAQ system [Maastricht-Programmable AcQuisition system (1000 Hz sample rate) with IDEEQ software Version 2.5; Maastricht Instruments, Maastricht, The Netherlands] allowing intraoperative monitoring of arterial blood pressure and heart rate. The surgical steps on the contralateral leg were identical, except that a Transonic flow probe (2RS; Transonic Systems Inc, Ithaca, New York, USA) was placed around the artery (Fig. 1e) and the iliotibialis, and semitendinosus muscle layers were sewn together over the flow probe (5-0 Mersilk, Ethicon) to hold it in place. The plug of the flow probe was exteriorised as described above. As the arterial catheter was already connected, we were able to record the cardiovascular response to lidocaine administration in the contralateral leg.
A custom-made CamChick jacket was then placed onto the chicken, to house the exteriorised catheters and flow probe cable when not attached to the M-PAQ system. Four stitches secured the jacket onto the skin to prevent it moving and displacing the catheters and flow probe lead. The chicken was then recovered from anaesthesia by gradually turning off the isoflorane but continuing the administration of oxygen and careful monitoring until consciousness was regained (Fig. 1f). The chicken was then administered Metacam (NSAID, 0.2 ml subcutaneous; Boehringer Ingelheim, Ingelheim am Rhein, Germany) and Baytril (broad-spectrum antibiotic, 0.8 ml intramuscular; Bayer Newbury, UK). Following surgery, birds were single housed but in sight of other birds, given a Buster collar (20 cm Buster birdcollar; Kruuse, Havretoften, Denmark) if necessary, and carefully observed until standing and eating.
Post-surgical care and daily maintenance
Complete weight bearing was observed between 2–24 h post-surgery. All catheters were flushed daily with heparinised saline (100 i.u./ml heparin in 0.9% NaCl) and an arterial blood sample (0.3 ml) was withdrawn daily to assess post-surgical blood gas, pH and metabolic health. An ABL5 blood analyzer (Radiometer; Copenhagen, measurements corrected to 42°C) was used to determine arterial blood pH, arterial partial pressure of oxygen (PaO 2 ), arterial partial pressure of carbon dioxide (PaCO2), bicarbonate concentration (HCO3 −) and calculated acid-base excess (ABE); an ABL80 blood gas analyzer (Radiometer, measurements corrected to 42°CReference Troxell, Petri and Daron 10 ) measured haemoglobin concentrations (Hb), percentage saturation of oxygen (HbO2), methaemoglobin concentrations (MetHb) and haematocrit; an automated glucose/lactate analyzer (Yellow Springs 2300 Stat Plus Glucose/Lactate Analyzer; YSI Ltd, Farnborough, UK) measured blood glucose and lactate concentrations. Chickens were given a daily dose of Metacam for the first 3 days following surgery (0.2 ml subcutaneous), and a daily dose of Baytril (0.8 ml intramuscular) for 7 days following surgery.
In vivo cardiovascular measurements
During cardiovascular recording, the arterial catheter was connected to a heparinised saline (100 i.u./ml heparin in 0.9% NaCl) filled pressure transducer (Argon Division, Maxxim Medical, Athens, TX, USA) via a blunt 19G needle and the flow probe was connected to a Transonic flow meter (T206; Transonic Systems Inc). Data were recorded via connection of the flow meter and the pressure transducer to an M-PAQ system [Maastricht-Programmable AcQuisition System (1000 Hz sample rate) with IDEEQ software Version 2.5]. Blood pressure was recorded both during surgery and following 5 days of recovery. Blood flow signals sometimes take longer to be able to be registered as the acoustic window encasing the vessel needs to be filled with generated tissue fluid for the electronic signal to be transmitted. In most cases, femoral blood flow could be recorded by the end of the recovery period. Heart rate was calculated continuously by IDEEQ using blood pressure as a trigger. Femoral vascular resistance (FVR) and femoral vascular conductance (FVC) were calculated from arterial blood pressure (ABP) and femoral blood flow (FBF) as an extension of Ohm’s law as follows [equations (1) and (2)]:
Heart rate (HR) responses to lidocaine were calculated using a stable baseline of around 2 min before the lidocaine dose, and a time period of 5 min after the lidocaine dose:
 $$\eqalignno { {\rm HR}\,{\rm relative}\,{\rm change}\,\left( {\rm \%} \right)\, \cr\quad{\rm {\equals} \,1}{\minus}\left( {\left( {{{{\rm lowest}\,{\rm HR}\,{\rm post{\hbox-}lidocaine}} \over {{\rm mean}\,{\rm HR}\,{\rm pre{\hbox-}lidocaine}}}} \right)} \right){\rm {\times}100}$$
$$\eqalignno { {\rm HR}\,{\rm relative}\,{\rm change}\,\left( {\rm \%} \right)\, \cr\quad{\rm {\equals} \,1}{\minus}\left( {\left( {{{{\rm lowest}\,{\rm HR}\,{\rm post{\hbox-}lidocaine}} \over {{\rm mean}\,{\rm HR}\,{\rm pre{\hbox-}lidocaine}}}} \right)} \right){\rm {\times}100}$$
Statistical analysis
All data are presented as mean±standard error of the mean (s.e.m.) unless otherwise stated, and comparisons were assessed for statistical significance using the Student’s t-test for paired data or one-way repeated measures analysis of variance as appropriate (Graphpad Prism: Version 5.00; Graphpad Software Inc, San Diego, CA, USA and SPSS: Version 23; IBM Analytics, New York, USA). For all comparisons, statistical significance was accepted when P<0.05.
Results
Anaesthesia caused a significant decrease in heart rate (Fig. 2a) as well as a significant reduction in mean arterial blood pressure (Fig. 2b), which was due to a reduction in diastolic pressure rather than systolic blood pressure (Fig. 2c and 2d). There was no significant effect on pulse pressure (Fig. 2e).
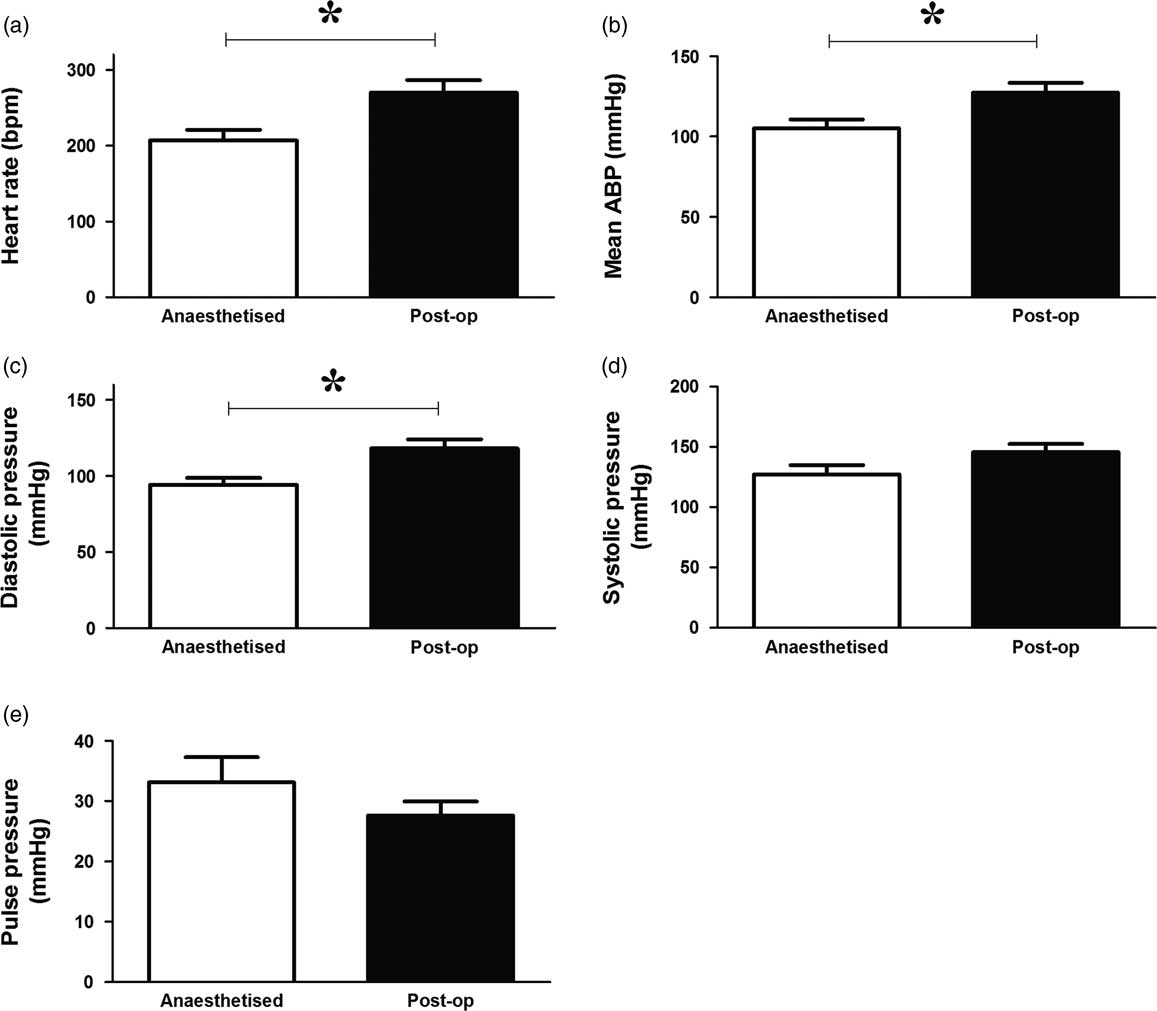
Fig. 2 Effects of anaesthesia on basal in vivo cardiovascular function in the adult chicken. Values are mean±s.e.m. for heart rate (a), mean arterial blood pressure (ABP; b), diastolic blood pressure (c), systolic blood pressure (d) and pulse pressure (e) under anaesthetic and postoperatively (after 5 days of recovery). n=7 chickens. *Represents a significant effect of anaesthesia (Student’s paired t-test, P<0.05).
Intramuscular administration of lidocaine had no significant effect on arterial blood pressure or pulse pressure, however, it consistently caused a decrease in heart rate (Table 1 and Fig. 3).
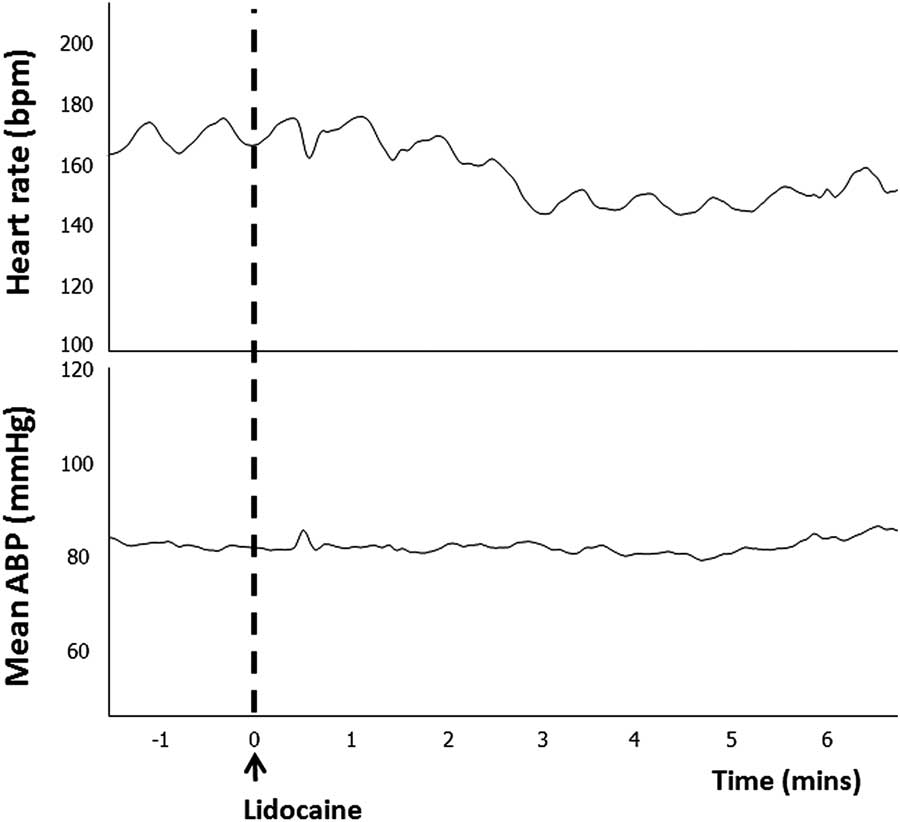
Fig. 3 Representative example of the in vivo cardiovascular response to lidocaine administration. Traces show heart rate and mean arterial blood pressure (ABP) response to a dose of lidocaine (arrow).
Table 1 Heart rate response to lidocaine

Values are presented for the absolute change in heart rate and the percentage change in heart rate following intramuscular administration of lidocaine. The final column displays mean changes±s.e.m.
During the post-surgical recovery period, there were no significant perturbations in chicken acid-base balance, haemoglobin, methaemoglobin or haematocrit levels, or blood glucose and lactate concentrations (Table 2). Typical values for adult chicken heart rate, arterial blood pressure, femoral vascular flow, resistance and conductance following full surgical recovery are shown in Table 3.
Table 2 Arterial blood gases during 5 days of surgical recovery
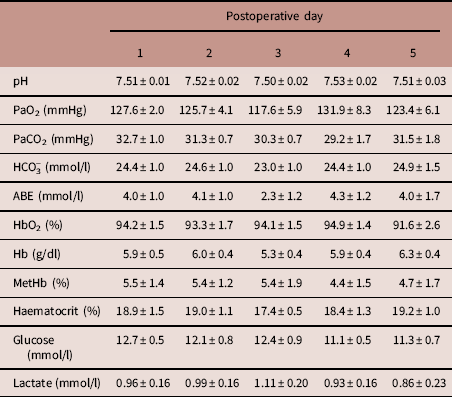
Mean±s.e.m. for descending aortic pH, partial pressure of oxygen (PaO2), partial pressure of carbon dioxide (PaCO2), bicarbonate concentration (HCO3 −), acid-base excess (ABE), haemoglobin saturation with oxygen (HbO2), haemoglobin concentration (Hb), methaemoglobin concentration (MetHb), haematocrit, glucose concentration and lactate concentration, measured daily for the first 5 days following surgery.
n=7 animals. There were no significant changes over time (one-way repeated measures analysis of variance).
Table 3 Basal in vivo cardiovascular function
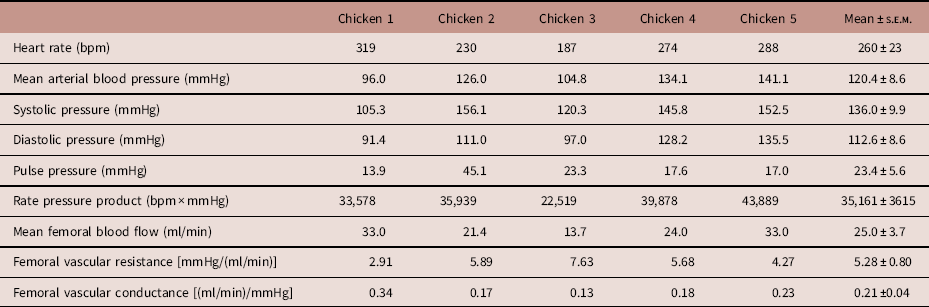
Values are presented for heart rate, mean arterial blood pressure, systolic pressure, diastolic pressure, pulse pressure, rate pressure product, mean femoral blood flow, mean femoral vascular resistance and mean femoral vascular conductance.
Values were obtained at a minimum of 5 days after surgery. The final column displays mean±s.e.m. across the five surgeries.
Discussion
This study established a surgical and experimental protocol for in vivo study of cardiovascular function in the adult chicken. The majority of animals were standing and eating within 12 h following surgery, and there were no significant blood gas perturbations. The blood volume of an adult chicken is estimated at 60 ml/kg,Reference Morton, Abbot and Barclay 11 and one of the most important factors for success was to minimise any blood loss during the surgery and recovery periods. We found that approaching the isolation of femoral vessels via the lateral thigh was more successful at minimising blood loss than via the inguinal approach. A junction between the iliotibialis and semitendinosus muscles could be easily found just superior to a fat pad, and the major vessels accessed easily by careful blunt dissection of the overlying muscles. We also found it helpful to place Gelfoam in the incision site just before suturing the wound closed. Occasionally, postoperative bleeding was suspected despite these precautions, and in this situation, we found it was often effective to apply pressure to the leg with a bandage overnight.
Other studies performing surgery on chickens including our own have utilised injectable anaesthetics, such as ketamine and xylazine.Reference Salinas, Blanco, Villena and Giussani 12 – Reference Ruijtenbeek, Kessels and Janssen 14 We found, however, that using isoflourane was satisfactory and permitted a much faster recovery within minutes. Anaesthesia in birds is notoriously difficult, and the animals must be carefully monitored for changes in heart rate, arterial blood oxygen saturation, breathing rate, comb colour and core body temperature. It is also extremely useful to connect the arterial catheter to a pressure transducer as soon as it is in place; not only does this allow accurate monitoring of arterial blood pressure and heart rate intraoperatively but is also useful to check the optimal positioning of the catheter via examination of the arterial pressure waveform. Lidocaine administration causes bradycardia via mechanisms including a reduction in cardiac pacemaker activity, decreased sympathetic nerve activity and reduced intraventricular conduction.Reference Carmeliet and Saikawa 15 – Reference Komai and Rusy 17 It is therefore especially important to monitor the birds immediately after the lidocaine administration.
Initial attempts were made to channel the catheters and flow probe lead subcutaneously to the origin of the wings, where they were exteriorised and housed in bags attached to Buster collars. However, the extra weight around the neck unbalanced the animals and prolonged the time duration to standing after surgery. Channelling the catheters and the flow probe lead only a short distance to the saddle of the chicken resulted in less bruising and a much faster recovery. Custom-made CamChick jackets were developed to house the catheters and the flow probe lead, and these were tolerated well by the chickens.
Normal values for blood gases and cardiovascular function of adult chickens can be found in Tables 2 and 3. It should be noted that they have higher basal body core temperature,Reference Troxell, Petri and Daron 10 higher basal arterial blood glucose levels, higher mean arterial blood pressure and higher heart rate measured in vivo compared with many mammalian species, differences which can in part be attributed to the high metabolic rate of avian species.Reference Holmes and Austad 18
The surgical protocols outlined permit in vivo study of the cardiovascular and metabolic health of post-hatching chickens. We found that chickens placed in a sling (so their feet do not touch the floor) in a dark box are very calm and mostly fall asleep, allowing easy obtainment of artefact-free recordings of cardiovascular function. Basal cardiovascular function can be measured (e.g. blood pressure, heart rate, heart rate variability), and placement of a flow probe additionally allows in vivo measurement of blood flow in circulations of interest, and associated calculation of vascular resistance and conductance.Reference Allison, Brain and Niu 19 , Reference Kane, Herrera, Camm and Giussani 20 Stimulated cardiovascular function can also be measured, for instance, the administration of increasing bolus doses of phenylephrine and angiotensin II permits investigation of well-known neuronal and endocrine vasoconstrictor mechanisms in vivo.Reference Kane, Herrera, Camm and Giussani 20 – Reference Thakor and Giussani 24 Similarly, administration of increasing bolus doses of sodium nitroprusside (SNP) permits investigation of nitric oxide-mediated vasodilator mechanisms in vivo.Reference Kane, Herrera, Camm and Giussani 20 , Reference Crossley, Hicks and Altimiras 23 Plotting the mean arterial pressure responses to infusion of pressor and depressor drugs against heart rate responses can be used to determine cardiac baroreflex function and investigate autonomic mechanisms in the control of in vivo cardiovascular function.Reference Herrera, Salinas, Blanco, Villena and Giussani 13 , Reference Kane, Herrera, Camm and Giussani 20 – Reference O’Connor, Ousey, Gardner, Fowden and Giussani 22 , Reference Thakor and Giussani 24 , Reference Altimiras and Crossley 25 FVR responses to SNP plotted against the fall in arterial blood pressure further permits determination of vasomotor baroreflex function in vivo.Reference Allison, Brain and Niu 26 Sustained infusion of SNP over 30 min can also be used to study the cardiovascular response to an acute hypotensive challenge.Reference Giussani, Forhead and Gardner 27 Furthermore, the nitric oxide clamp techniqueReference Gardner, Powlson and Giussani 28 , Reference Gardner and Giussani 29 can be used to assess the contribution of nitric oxide to the cardiovascular response to pressor and depressor drugs. Blood samples can also be taken during basal and stimulated conditions for subsequent measurement of endocrine responses to challenges, for instance changes in circulating hormones such as catecholamines, adrenocorticotrophic hormone and cortisol, to address changes in the stress axis.Reference O’Connor, Gardner and Ousey 30 – Reference Thakor and Giussani 32 The avian model could also be used to perform glucose or insulin tolerance tests to determine metabolic function.Reference Musial, Vaughan and Fernandez-Twinn 33
Developmental programming of disease is a fast-moving field, and there is much interest in determining the effects of common adverse conditions during development such as maternal obesity,Reference Gaillard 34 exposure to stressReference Cottrell and Seckl 35 or chronic fetal hypoxiaReference Giussani, Camm and Niu 36 on cardiovascular function in adult offspring. The chicken permits isolation of these effects on the physiology of the offspring, independent of effects via the mother and placenta and is therefore a powerful animal model to enhance the understanding of the field of mechanisms mediating fetal programming.
Acknowledgements
D.A.G. is the Professor of Cardiovascular Developmental Physiology & Medicine at the Department of Physiology Development & Neuroscience at the University of Cambridge, Professorial Fellow and Director of Studies in Medicine at Gonville & Caius College, a Lister Institute Fellow and a Royal Society Wolfson Research Merit Award Holder.
Financial Support
This work was supported by the British Heart Foundation.
Conflicts of Interest
None.
Ethical Standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national guides on the care and use of laboratory animals [the UK Animals (Scientific Procedures) Act 1986] and has been approved by the institutional committee (Local Ethics Review Committee of the University of Cambridge).








