Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by
Crossref.
Beesoon, Sanjay
Behary, Nemeshwaree
and
Perwuelz, Anne
2020.
Universal masking during COVID-19 pandemic: Can textile engineering help public health? Narrative review of the evidence.
Preventive Medicine,
Vol. 139,
Issue. ,
p.
106236.
Wang, Hongping
Li, Zhaobin
Zhang, Xinlei
Zhu, Lixing
Liu, Yi
and
Wang, Shizhao
2020.
The motion of respiratory droplets produced by coughing.
Physics of Fluids,
Vol. 32,
Issue. 12,
Murphy, J. H.
2020.
Personal protective equipment during the COVID‐19 pandemic: a comment.
Anaesthesia,
Vol. 75,
Issue. 8,
p.
1121.
Long, Kenneth D.
Woodburn, Elizabeth V.
Berg, Ian C.
Chen, Valerie
Scott, William S.
and
Zhang, Ming
2020.
Measurement of filtration efficiencies of healthcare and consumer materials using modified respirator fit tester setup.
PLOS ONE,
Vol. 15,
Issue. 10,
p.
e0240499.
Cummins, C. P.
Ajayi, O. J.
Mehendale, F. V.
Gabl, R.
and
Viola, I. M.
2020.
The dispersion of spherical droplets in source–sink flows and their relevance to the COVID-19 pandemic.
Physics of Fluids,
Vol. 32,
Issue. 8,
Taylor, Charles A.
Boulos, Christopher
and
Almond, Douglas
2020.
Livestock plants and COVID-19 transmission.
Proceedings of the National Academy of Sciences,
Vol. 117,
Issue. 50,
p.
31706.
Romero, Van
Stone, William D.
and
Ford, Julie Dyke
2020.
COVID-19 indoor exposure levels: An analysis of foot traffic scenarios within an academic building.
Transportation Research Interdisciplinary Perspectives,
Vol. 7,
Issue. ,
p.
100185.
Zhao, Lei
Qi, Yuhang
Luzzatto-Fegiz, Paolo
Cui, Yi
and
Zhu, Yangying
2020.
COVID-19: Effects of Environmental Conditions on the Propagation of Respiratory Droplets.
Nano Letters,
Vol. 20,
Issue. 10,
p.
7744.
Abkarian, M.
and
Stone, H. A.
2020.
Stretching and break-up of saliva filaments during speech: A route for pathogen aerosolization and its potential mitigation.
Physical Review Fluids,
Vol. 5,
Issue. 10,
Din, Ahmed Riaz
Hindocha, Annika
Patel, Tulsi
Sudarshan, Sanjana
Cagney, Neil
Koched, Amine
Mueller, Jens-Dominik
Seoudi, Noha
Morgan, Claire
Shahdad, Shakeel
and
Fleming, Padhraig S.
2020.
Quantitative analysis of particulate matter release during orthodontic procedures: a pilot study.
British Dental Journal,
Chaudhuri, Swetaprovo
Basu, Saptarshi
and
Saha, Abhishek
2020.
Analyzing the dominant SARS-CoV-2 transmission routes toward an ab initio disease spread model.
Physics of Fluids,
Vol. 32,
Issue. 12,
Wong, David W. S.
Li, Yun
and
Xue, Bing
2020.
Spreading of COVID-19: Density matters.
PLOS ONE,
Vol. 15,
Issue. 12,
p.
e0242398.
Weiss, Philipp
Giddey, Valentin
Meyer, Daniel W.
and
Jenny, Patrick
2020.
Evaporating droplets in shear turbulence.
Physics of Fluids,
Vol. 32,
Issue. 7,
Ching, Joseph
and
Kajino, Mizuo
2020.
Rethinking Air Quality and Climate Change after COVID-19.
International Journal of Environmental Research and Public Health,
Vol. 17,
Issue. 14,
p.
5167.
Mittal, Rajat
Meneveau, Charles
and
Wu, Wen
2020.
A mathematical framework for estimating risk of airborne transmission of COVID-19 with application to face mask use and social distancing.
Physics of Fluids,
Vol. 32,
Issue. 10,
p.
101903.
Zhou, Jiayi
and
Andersson, Mats
2020.
An analysis of surface breakup induced by laser-generated cavitation bubbles in a turbulent liquid jet.
Experiments in Fluids,
Vol. 61,
Issue. 12,
Vuorinen, Ville
Aarnio, Mia
Alava, Mikko
Alopaeus, Ville
Atanasova, Nina
Auvinen, Mikko
Balasubramanian, Nallannan
Bordbar, Hadi
Erästö, Panu
Grande, Rafael
Hayward, Nick
Hellsten, Antti
Hostikka, Simo
Hokkanen, Jyrki
Kaario, Ossi
Karvinen, Aku
Kivistö, Ilkka
Korhonen, Marko
Kosonen, Risto
Kuusela, Janne
Lestinen, Sami
Laurila, Erkki
Nieminen, Heikki J.
Peltonen, Petteri
Pokki, Juho
Puisto, Antti
Råback, Peter
Salmenjoki, Henri
Sironen, Tarja
and
Österberg, Monika
2020.
Modelling aerosol transport and virus exposure with numerical simulations in relation to SARS-CoV-2 transmission by inhalation indoors.
Safety Science,
Vol. 130,
Issue. ,
p.
104866.
Das, Santosh K.
Alam, Jan-e
Plumari, Salvatore
and
Greco, Vincenzo
2020.
Transmission of airborne virus through sneezed and coughed droplets.
Physics of Fluids,
Vol. 32,
Issue. 9,
Lipinski, Tom
Ahmad, Darem
Serey, Nicolas
and
Jouhara, Hussam
2020.
Review of ventilation strategies to reduce the risk of disease transmission in high occupancy buildings.
International Journal of Thermofluids,
Vol. 7-8,
Issue. ,
p.
100045.
Zhang, Zhijie
Arshad, Arfan
Zhang, Chuanrong
Hussain, Saddam
and
Li, Weidong
2020.
Unprecedented Temporary Reduction in Global Air Pollution Associated with COVID-19 Forced Confinement: A Continental and City Scale Analysis.
Remote Sensing,
Vol. 12,
Issue. 15,
p.
2420.
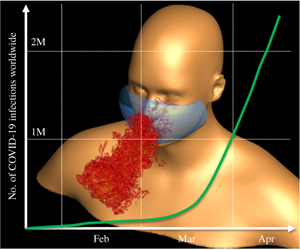
1 Introduction
Transmission of respiratory infections such as COVID-19 is primarily via virus-laden fluid particles (i.e. droplets and aerosols) that are formed in the respiratory tract of an infected person and expelled from the mouth and nose during breathing, talking, coughing and sneezing (Jones & Brosseau Reference Jones and Brosseau2015; Asadi et al. Reference Asadi, Bouvier, Wexler and Ristenpart2020; Bourouiba Reference Bourouiba2020; CDC 2020a). Wells (Reference Wells1934, Reference Wells1955) showed that the competing effects of inertia, gravity and evaporation determine the fate of these droplets. Droplets larger than a critical size settle faster than they evaporate, and so contaminate surrounding surfaces. Droplets smaller than this size evaporate faster than they settle, so forming droplet nuclei that can stay airborne for hours and may be transported over long distances.
Human-to-human transmission of COVID-19 occurs primarily via three routes: large droplets that are expelled with sufficient momentum so as to directly impact the recipients’ mouth, nose or conjunctiva; physical contact with droplets deposited on a surface and subsequent transfer to the recipient’s respiratory mucosa; and inhalation by the recipient of aerosolized droplet nuclei from the expiratory ejecta that are delivered by ambient air currents. The first two routes associated with large droplets are referred to as the ‘droplet’ and ‘contact’ routes of transmission, whereas the third is the so-called ‘airborne’ transmission route (Jones & Brosseau Reference Jones and Brosseau2015). Respiratory infections hijack our respiratory apparatus to increase the frequency and intensity of expiratory events, such as coughing and sneezing, which are particularly effective in generating and dispersing virus-carrying droplets.
Each stage in the transmission process is mediated by complex flow phenomena, ranging from air–mucous interaction, liquid sheet fragmentation, turbulent jets, and droplet evaporation and deposition, to flow-induced particle dispersion and sedimentation. Thus, flow physics is central to the transmission of COVID-19. Furthermore, given the importance of flow phenomena to the transmission process, the methods, devices and practices employed to mitigate respiratory infections are also rooted in the principles of fluid dynamics. These include simple methods such as hand washing and wearing face masks, to fogging machines, ventilation (Tang et al. Reference Tang, Li, Eames, Chan and Ridgway2006) and even practices such as social distancing. However, despite the long history of medical research and experience in the transmission of respiratory infections (a fascinating account of the ‘Spanish flu’ can be found in Soper (Reference Soper1919)), the rapid advance of COVID-19 around the world has laid bare the limits of our knowledge regarding the physics underlying the transmission process, as well as the inadequacy of the methods, devices and practices used to curtail transmission rates. For instance, one factor that is contributing to the rapid growth of COVID-19 infections is the higher viral load of the SARS-CoV-2 virus in the upper respiratory tract of asymptomatic hosts who shed virus-laden droplets during normal activities such as talking and breathing (Bai et al. Reference Bai, Yao, Wei, Tian, Jin, Chen and Wang2020). This knowledge gap has also manifested through guidelines on practices such as social distancing and the wearing of face masks (Dwyer & Aubrey Reference Dwyer and Aubrey2020; Elegant Reference Elegant2020), which are based on outdated science (Asadi et al. Reference Asadi, Bouvier, Wexler and Ristenpart2020; Bourouiba Reference Bourouiba2020).
This article attempts to summarize our current state of knowledge regarding the flow physics implicated in the transmission of COVID-19. The challenge of summarizing such a vast topic is amplified by the need of the hour, that speed take precedence over comprehensiveness. Readers are therefore referred to other articles on this topic (Tang et al. Reference Tang, Li, Eames, Chan and Ridgway2006; Xie et al. Reference Xie, Li, Chwang, Ho and Seto2007; Johnson & Morawska Reference Johnson and Morawska2009; Tang et al. Reference Tang, Liebner, Craven and Settles2009; Jones & Brosseau Reference Jones and Brosseau2015; Asadi et al. Reference Asadi, Bouvier, Wexler and Ristenpart2020; Bourouiba Reference Bourouiba2020) to fill the many gaps that are sure to be left by this article.
2 Respiratory droplets and aerosols
This section addresses the generation, expulsion, evolution and transport of droplets and aerosols generated from the respiratory tract during expiratory activities such as breathing, talking, coughing and sneezing. The primary objective of fluid dynamic analyses in this setting is to: (a) determine the mechanisms for the generation of these droplets within the respiratory tract; (b) characterize the number density, size distribution and velocity of ejected droplets; (c) determine the critical droplet size for transition between the large and small droplet transmission routes; (d) estimate the settling distance of large droplets; (e) determine the evaporation times of small droplets; (f) characterize the transport of small droplets and droplet nuclei in the air; and (g) quantify the effect of external factors such as air currents, temperature and humidity on all of the above.
2.1 Mechanisms of droplet formation
It is generally established that respiratory droplets are formed from the fluid lining of the respiratory tract (Edwards et al. Reference Edwards, Man, Brand, Katstra, Sommerer, Stone, Nardell and Scheuch2004; Morawska et al. Reference Morawska, Hofmann, Hitchins-Loveday, Swanson and Mengersen2005, Reference Morawska, Johnson, Ristovski, Hargreaves, Mengersen, Corbett, Chao, Li and Katoshevski2009; Johnson & Morawska Reference Johnson and Morawska2009; Almstrand et al. Reference Almstrand, Bake, Ljungström, Larsson, Bredberg, Mirgorodskaya and Olin2010). The mechanisms of formation are usually associated with distinct locations in the respiratory tract; this is important because both the characteristics of the respiratory tract (length scales, airway elasticity, mucus and saliva properties, etc.) as well as the viral load carried by the lining are functions of the location (Almstrand et al. Reference Almstrand, Bake, Ljungström, Larsson, Bredberg, Mirgorodskaya and Olin2010; Johnson et al. Reference Johnson, Morawska, Ristovski, Hargreaves, Mengersen, Chao, Wan, Li, Xie and Katoshevski2011).
One key mechanism for the generation of respiratory droplets is the instability (Moriarty & Grotberg Reference Moriarty and Grotberg1999) and eventual fragmentation of the mucus lining due to the shear stress induced by the airflow. Predicting the fragmentation and droplet size distribution resulting from this fragmentation is non-trivial because mucus is a viscoelastic shear-thinning fluid subject to surface tension. This enables multiple instabilities to bear on this problem (Malashenko, Tsuda & Haber Reference Malashenko, Tsuda and Haber2009), including surface-tension-driven Rayleigh–Plateau instability (Eggers Reference Eggers1997; Lin & Reitz Reference Lin and Reitz1998; Romanò et al. Reference Romanò, Fujioka, Muradoglu and Grotberg2019), shear-driven Kelvin–Helmholtz instability (Kataoka, Ishii & Mishima Reference Kataoka, Ishii and Mishima1983; Scardovelli & Zaleski Reference Scardovelli and Zaleski1999) and acceleration-driven Rayleigh–Taylor instability (Joseph, Beavers & Funada Reference Joseph, Beavers and Funada2002; Halpern & Grotberg Reference Halpern and Grotberg2003). The Rayleigh–Taylor instability is particularly important in spasmodic events such as coughing and sneezing.
The second mechanism for droplet formation is associated with the rupture of the fluid lining during the opening of a closed respiratory passage (Malashenko et al. Reference Malashenko, Tsuda and Haber2009). One important site for this mechanism is in the terminal bronchioles during breathing. These submillimetre-sized passages collapse during exhalation, and the subsequent reopening during inhalation ruptures the mucus meniscus, resulting in the generation of micrometre-sized droplets (Almstrand et al. Reference Almstrand, Bake, Ljungström, Larsson, Bredberg, Mirgorodskaya and Olin2010; Johnson et al. Reference Johnson, Morawska, Ristovski, Hargreaves, Mengersen, Chao, Wan, Li, Xie and Katoshevski2011). A similar mechanism probably occurs in the larynx during activities such as talking and coughing, which involve the opening and closing of the vocal folds (Mittal, Erath & Plesniak Reference Mittal, Erath and Plesniak2013). Finally, movement and contact of the tongue and lips, particularly during violent events such as sneezing, generate salivary droplets via this mechanism. The fluid dynamics of meniscus breakup associated with this mechanism is difficult to predict, especially given the non-Newtonian properties of the fluids involved, the dominant role of moving boundaries, and the large range of length and time scales implicated in this phenomenon.
2.2 Droplet characteristics
The number density, velocity and size distributions of droplets ejected by expiratory events have important implications for transmission, and numerous studies have attempted to measure these characteristics (Duguid Reference Duguid1946; Wells Reference Wells1955; Morawska et al. Reference Morawska, Johnson, Ristovski, Hargreaves, Mengersen, Corbett, Chao, Li and Katoshevski2009; Xie et al. Reference Xie, Li, Sun and Liu2009; Han, Weng & Huang Reference Han, Weng and Huang2013; Bourouiba, Dehandschoewercker & Bush Reference Bourouiba, Dehandschoewercker and Bush2014; Scharfman et al. Reference Scharfman, Techet, Bush and Bourouiba2016; Asadi et al. Reference Asadi, Wexler, Cappa, Barreda, Bouvier and Ristenpart2019). A single sneeze can generate $O(10^{4})$ or more droplets, with velocities upwards of
$O(10^{4})$ or more droplets, with velocities upwards of  $20~\text{m}~\text{s}^{-1}$ (Han et al. Reference Han, Weng and Huang2013). Coughing generates 10–100 times fewer droplets than sneezing, with velocities of approximately
$20~\text{m}~\text{s}^{-1}$ (Han et al. Reference Han, Weng and Huang2013). Coughing generates 10–100 times fewer droplets than sneezing, with velocities of approximately  $10~\text{m}~\text{s}^{-1}$, but even talking can generate approximately 50 particles per second (Asadi et al. Reference Asadi, Wexler, Cappa, Barreda, Bouvier and Ristenpart2019). Measured droplet sizes range over four orders of magnitude, from
$10~\text{m}~\text{s}^{-1}$, but even talking can generate approximately 50 particles per second (Asadi et al. Reference Asadi, Wexler, Cappa, Barreda, Bouvier and Ristenpart2019). Measured droplet sizes range over four orders of magnitude, from  $O(0.1)$ to
$O(0.1)$ to  $O(1000)~\unicode[STIX]{x03BC}\text{m}$. Recent studies have noted that, while breathing generates droplets at a much lower rate, it probably accounts for more expired bioaerosols over the course of a day than intermittent events such as coughing and sneezing (Fiegel, Clarke & Edwards Reference Fiegel, Clarke and Edwards2006; Atkinson & Wein Reference Atkinson and Wein2008). Consensus on all these droplet characteristics continues to be elusive due to the multifactorial nature of the phenomena as well as the difficulty of making such measurements (Chao et al. Reference Chao, Wan, Morawska, Johnson, Ristovski, Hargreaves, Mengersen, Corbett, Li, Xie and Katoshevski2009; Morawska et al. Reference Morawska, Johnson, Ristovski, Hargreaves, Mengersen, Corbett, Chao, Li and Katoshevski2009; Han et al. Reference Han, Weng and Huang2013).
$O(1000)~\unicode[STIX]{x03BC}\text{m}$. Recent studies have noted that, while breathing generates droplets at a much lower rate, it probably accounts for more expired bioaerosols over the course of a day than intermittent events such as coughing and sneezing (Fiegel, Clarke & Edwards Reference Fiegel, Clarke and Edwards2006; Atkinson & Wein Reference Atkinson and Wein2008). Consensus on all these droplet characteristics continues to be elusive due to the multifactorial nature of the phenomena as well as the difficulty of making such measurements (Chao et al. Reference Chao, Wan, Morawska, Johnson, Ristovski, Hargreaves, Mengersen, Corbett, Li, Xie and Katoshevski2009; Morawska et al. Reference Morawska, Johnson, Ristovski, Hargreaves, Mengersen, Corbett, Chao, Li and Katoshevski2009; Han et al. Reference Han, Weng and Huang2013).
2.3 The expiratory jet and droplet transmission
Droplets generated within the respiratory tract by the mechanisms described above are carried outwards by the respiratory airflow, and those droplets that are not reabsorbed by the fluid lining are expelled within a two-phase buoyant jet from the mouth and/or nose. Breathing and talking generate jet velocities that seldom exceed $5~\text{m}~\text{s}^{-1}$ (Tang et al. Reference Tang, Nicolle, Klettner, Pantelic, Wang, Suhaimi, Tan, Ong, Su and Sekhar2013) and mostly expel small droplets. Violent expiratory events like coughing and sneezing, on the other hand, generate turbulent jets with Reynolds numbers of
$5~\text{m}~\text{s}^{-1}$ (Tang et al. Reference Tang, Nicolle, Klettner, Pantelic, Wang, Suhaimi, Tan, Ong, Su and Sekhar2013) and mostly expel small droplets. Violent expiratory events like coughing and sneezing, on the other hand, generate turbulent jets with Reynolds numbers of  $O(10^{4})$ and higher (Bourouiba et al. Reference Bourouiba, Dehandschoewercker and Bush2014). Mucus and saliva that are expelled out of the nose and mouth can be stretched into ligaments and sheets, and eventually fragment into small droplets if the Weber number is large enough (Jain et al. Reference Jain, Prakash, Tomar and Ravikrishna2015). This breakup process probably contributes to the generation of large droplets that fall ballistically and contaminate nearby surfaces (Bourouiba et al. Reference Bourouiba, Dehandschoewercker and Bush2014).
$O(10^{4})$ and higher (Bourouiba et al. Reference Bourouiba, Dehandschoewercker and Bush2014). Mucus and saliva that are expelled out of the nose and mouth can be stretched into ligaments and sheets, and eventually fragment into small droplets if the Weber number is large enough (Jain et al. Reference Jain, Prakash, Tomar and Ravikrishna2015). This breakup process probably contributes to the generation of large droplets that fall ballistically and contaminate nearby surfaces (Bourouiba et al. Reference Bourouiba, Dehandschoewercker and Bush2014).
Wells’ simple but elegant analysis predicted that the critical size that differentiates large from small droplets is approximately $100~\unicode[STIX]{x03BC}\text{m}$ (Wells Reference Wells1934). Subsequent analysis has shown that typical temperature and humidity variations expand the critical size range from approximately
$100~\unicode[STIX]{x03BC}\text{m}$ (Wells Reference Wells1934). Subsequent analysis has shown that typical temperature and humidity variations expand the critical size range from approximately  $50$ to
$50$ to  $150~\unicode[STIX]{x03BC}\text{m}$ (Xie et al. Reference Xie, Li, Chwang, Ho and Seto2007). For the droplet transmission route, an important consideration is the horizontal distance travelled by the large droplets. Thus the 3–6 feet social distancing guidelines (CDC 2020b; WHO 2020) probably originate from Wells’ original work. However, studies indicate that, while this distance might be adequate for droplets expelled during breathing and coughing (Xie et al. Reference Xie, Li, Chwang, Ho and Seto2007; Wei & Li Reference Wei and Li2015), large droplets expelled from sneezes may travel 20 feet or more (Xie et al. Reference Xie, Li, Chwang, Ho and Seto2007; Bourouiba et al. Reference Bourouiba, Dehandschoewercker and Bush2014). Studies also suggest that social distancing in indoor environments (Wong et al. Reference Wong, Lee, Tam, Lau, Yu, Lui, Chan, Li, Bresee and Sung2004) could be complicated by ventilation-system-induced air currents.
$150~\unicode[STIX]{x03BC}\text{m}$ (Xie et al. Reference Xie, Li, Chwang, Ho and Seto2007). For the droplet transmission route, an important consideration is the horizontal distance travelled by the large droplets. Thus the 3–6 feet social distancing guidelines (CDC 2020b; WHO 2020) probably originate from Wells’ original work. However, studies indicate that, while this distance might be adequate for droplets expelled during breathing and coughing (Xie et al. Reference Xie, Li, Chwang, Ho and Seto2007; Wei & Li Reference Wei and Li2015), large droplets expelled from sneezes may travel 20 feet or more (Xie et al. Reference Xie, Li, Chwang, Ho and Seto2007; Bourouiba et al. Reference Bourouiba, Dehandschoewercker and Bush2014). Studies also suggest that social distancing in indoor environments (Wong et al. Reference Wong, Lee, Tam, Lau, Yu, Lui, Chan, Li, Bresee and Sung2004) could be complicated by ventilation-system-induced air currents.
It has also been shown that the respiratory jet transforms into a turbulent cloud or puff (Bourouiba et al. Reference Bourouiba, Dehandschoewercker and Bush2014). While large droplets are mostly not affected by the cloud dynamics, small and medium-sized droplets can be suspended in the turbulent cloud for a longer time by its circulatory flow, thereby extending the travel distance significantly (Bourouiba et al. Reference Bourouiba, Dehandschoewercker and Bush2014). This also has important implications for transmission via indirect contact with contaminated surfaces, since SARS-CoV-2 is able to survive on many types of surfaces for hours (van Doremalen et al. Reference van Doremalen, Bushmaker, Morris, Holbrook, Gamble, Williamson, Tamin, Harcourt, Thornburg and Gerber2020). The turbulent cloud also moves upwards due to buoyancy (Bourouiba et al. Reference Bourouiba, Dehandschoewercker and Bush2014), thereby enabling small droplets and droplet nuclei to reach heights where they can enter the ventilation system and accelerate airborne transmissions (see § 2.5). The notion of a critical droplet size that was introduced by Wells (Reference Wells1934) might need to be re-examined in the light of our rapidly evolving knowledge about these expiratory events (Xie et al. Reference Xie, Li, Chwang, Ho and Seto2007; Bourouiba et al. Reference Bourouiba, Dehandschoewercker and Bush2014).
2.4 Droplet evaporation and droplet nuclei
Droplet evaporation plays a singularly important role in the eventual fate of a droplet (Wells Reference Wells1934). The rate of evaporation depends on the difference between the droplet surface saturation vapour pressure and the vapour pressure of the surrounding air, the latter being dependent on humidity. The evaporation rate also depends on the mass-diffusion coefficient, which is a strong function of surface-to-ambient temperature difference, as well as the relative velocity between the droplet and surrounding gas. Thus, Reynolds, Nusselt and Sherwood numbers for the droplets are just some of the non-dimensional parameters that determine this phenomenon (Xie et al. Reference Xie, Li, Chwang, Ho and Seto2007). This dependence of evaporation rates on the ambient temperature and humidity has implications for the very important, and as yet unresolved, questions regarding seasonal and geographic variations in transmission rates (Tang Reference Tang2009; Ma et al. Reference Ma, Zhao, Liu, He, Wang, Fu, Yan, Niu, Zhou and Luo2020) as well as transmission in various indoor environments (Tang et al. Reference Tang, Li, Eames, Chan and Ridgway2006; Li et al. Reference Li, Leung, Tang, Yang, Chao, Lin, Lu, Nielsen, Niu and Qian2007).
Higher temperatures and lower relative humidities lead to larger evaporation rates that increase the critical droplet size (Wells Reference Wells1934; Xie et al. Reference Xie, Li, Chwang, Ho and Seto2007). However, temperature changes are usually accompanied by changes in humidity, and the overall effect of environmental conditions on transmission rates has been difficult to ascertain. This is not only due to the fact that these factors modulate the relative importance of the droplet and airborne routes of transmission, but also because survivability of enveloped viruses such as SARS-CoV-2 seem to be linked to these factors in a complex, non-monotonic manner (Geller, Varbanov & Duval Reference Geller, Varbanov and Duval2012). Models that can combine droplet/aerosol fluid dynamics with virus microbiology and/or population dynamics could help unravel this complex effect of ambient conditions on transmission rates.
2.5 Airborne transmission
The airborne transmission route is associated with small droplets that are suspended and transported in air currents. Most of these droplets evaporate within a few seconds (Xie et al. Reference Xie, Li, Chwang, Ho and Seto2007) to form droplet nuclei, although the vapour-rich, buoyant turbulent expiratory jet can slow this evaporation process (Bourouiba Reference Bourouiba2020). The nuclei consist of virions and solid residue (Vejerano & Marr Reference Vejerano and Marr2018), but water may never be completely removed (Mezhericher, Levy & Borde Reference Mezhericher, Levy and Borde2010). These droplet nuclei are submicrometre to approximately $10~\unicode[STIX]{x03BC}\text{m}$ in size, and may remain suspended in the air for hours. Each droplet nucleus could contain multiple virions, and, given the approximately one hour viability half-life of the SARS-CoV-2 virus (van Doremalen et al. Reference van Doremalen, Bushmaker, Morris, Holbrook, Gamble, Williamson, Tamin, Harcourt, Thornburg and Gerber2020) and the fact that SARS-type infections in a host may potentially be caused by a single virus (Nicas, Nazaroff & Hubbard Reference Nicas, Nazaroff and Hubbard2005), droplet nuclei play a singularly important role in the transmission of COVID-19-type infections (Asadi et al. Reference Asadi, Bouvier, Wexler and Ristenpart2020). The evaporation process of virus-laden respiratory droplets and the composition of droplet nuclei require further analysis because these have implications for the viability and potency of the virus that is transported by these nuclei.
$10~\unicode[STIX]{x03BC}\text{m}$ in size, and may remain suspended in the air for hours. Each droplet nucleus could contain multiple virions, and, given the approximately one hour viability half-life of the SARS-CoV-2 virus (van Doremalen et al. Reference van Doremalen, Bushmaker, Morris, Holbrook, Gamble, Williamson, Tamin, Harcourt, Thornburg and Gerber2020) and the fact that SARS-type infections in a host may potentially be caused by a single virus (Nicas, Nazaroff & Hubbard Reference Nicas, Nazaroff and Hubbard2005), droplet nuclei play a singularly important role in the transmission of COVID-19-type infections (Asadi et al. Reference Asadi, Bouvier, Wexler and Ristenpart2020). The evaporation process of virus-laden respiratory droplets and the composition of droplet nuclei require further analysis because these have implications for the viability and potency of the virus that is transported by these nuclei.
The transport of droplet nuclei over larger distances is primarily driven by ambient flows, and indoor environments such as homes, offices, malls, aircraft and public transport vehicles pose a particular challenge for disease transmission. The importance of ventilation in controlling airborne transmission of infections is well known (Tang et al. Reference Tang, Li, Eames, Chan and Ridgway2006; Li et al. Reference Li, Leung, Tang, Yang, Chao, Lin, Lu, Nielsen, Niu and Qian2007) and much of the recent work in this area has exploited the power of computational fluid dynamic (CFD) modelling (Thatiparti, Ghia & Mead Reference Thatiparti, Ghia and Mead2017; Yang et al. Reference Yang, Li, Yan and Tu2018; Yu, Mui & Wong Reference Yu, Mui and Wong2018). However, indoor spaces can have extremely complex flows, due not only to the presence of recirculatory flows driven by ventilation systems but also to anthropogenic thermally driven flow effects (Craven & Settles Reference Craven and Settles2006; Licina et al. Reference Licina, Pantelic, Melikov, Sekhar and Tham2014). COVID-19 transmission from asymptomatic hosts (Bai et al. Reference Bai, Yao, Wei, Tian, Jin, Chen and Wang2020; Ye et al. Reference Ye, Xu, Rong, Xu, Liu, Deng, Liu and Xu2020) makes it more critical than ever that we develop methods of analysis that provide better prediction of these effects.
3 Inhalation and deposition of bioaerosols
The process of inhalation of virus-laden particles/droplets and deposition of the virus in the respiratory mucosa of the host is the final stage of airborne transmission. Fortunately, particle transport and deposition in the human airway has been studied extensively in the context of drug delivery (Heyder Reference Heyder2004), food smell (Ni et al. Reference Ni, Michalski, Brown, Doan, Zinter, Ouellette and Shepherd2015) and pollutant transport (Morawska et al. Reference Morawska, Hofmann, Hitchins-Loveday, Swanson and Mengersen2005). The deposition of a solid particle is governed primarily by the mechanism of transport, whereas for liquid aerosols the evaporation/diffusion process contributes significantly to the deposition mechanism. The latter is, however, a complex subject and has not been studied extensively so far (Rostami Reference Rostami2009). There are six mechanisms that determine the deposition location: impaction, sedimentation, interception, diffusion, electrostatic precipitation and convection (Hinds Reference Hinds1999). The relative importance of these mechanisms depends on the particle size and the region of the airway where deposition occurs. In general, for larger particles, impaction, sedimentation and interception are more important than diffusion and convection (Rostami Reference Rostami2009). For droplet-nuclei-sized particles, sedimentation will drive significant deposition in the upper respiratory tract of the host (Willeke, Baron & Martonen Reference Willeke, Baron and Martonen1993).
Deposition of virus-bearing droplets in the respiratory tract does not always result in infection, since the mucus layer provides some level of protection against virus invasion and subsequent infection (Zanin et al. Reference Zanin, Baviskar, Webster and Webby2016). The rate of droplet/nuclei deposition in the respiratory tract is quantified by the non-dimensionalized deposition velocity (Friedlander & Johnstone Reference Friedlander and Johnstone1957; Liu & Agarwal Reference Liu and Agarwal1974), which can vary by over four orders of magnitude (Guha Reference Guha2008). For small droplets, deposition relies completely on turbulent diffusion (Friedlander & Johnstone Reference Friedlander and Johnstone1957), but for large droplets, the deposition velocity increases substantially due to impact on the highly curved and complex passage walls of the respiratory tract. Large droplets, despite a higher deposition velocity, probably deposit in the upper respiratory system, and could be deactivated by the first defensive layer of the mucosa (Fokkens & Scheeren Reference Fokkens and Scheeren2000). On the other hand, droplet nuclei, despite their smaller deposition velocity, will penetrate deeper into the respiratory system, and this could affect the progression and intensity of the infection.
Imaging modalities such as computed tomography (CT) and magnetic resonance imaging (MRI) provide realistic anatomical models for experiments (Ni et al. Reference Ni, Michalski, Brown, Doan, Zinter, Ouellette and Shepherd2015) and CFD models (Rostami Reference Rostami2009), from which local deposition can be quantified. A recent study even included the immune system response in the model (Haghnegahdar, Zhao & Feng Reference Haghnegahdar, Zhao and Feng2019), and similar models that combine fluid dynamics, biomechanics and virology could serve as important tools in combating such pandemics.
4 Measures to mitigate transmission
4.1 Mucus property modification
The physical properties of the mucus play a key role in droplet formation within the respiratory tract. Transient modification of the physical properties of the mucus lining via material delivery to enhance mucus stability therefore provides a means for reducing infection rates. Fiegel et al. (Reference Fiegel, Clarke and Edwards2006) used isotonic saline to change the mucus lining properties via the induced ionic charge to reduce droplet formation, and Edwards et al. (Reference Edwards, Man, Brand, Katstra, Sommerer, Stone, Nardell and Scheuch2004) explored the use of surface-tension-enhancing inhalants to reduce droplet generation. These techniques involve complex multiphysics flow phenomena that could benefit from advanced experimental and computational techniques.
4.2 Fogging machines
Fogging machines provide an effective means for disinfecting large spaces, such as hospitals, nursing homes, grocery stores and airplanes. Fogging machines that rely on the dispersion of a fine mist of disinfectants in the air have proven their performance in the healthcare sector (Otter et al. Reference Otter, Yezli, Perl, Barbut and French2013) and the food industry (Oh et al. Reference Oh, Gray, Dougherty and Kang2005). Commercial fogging machines are also designed based on the same flow physics of aerosolization, and their droplet size is below $10~\unicode[STIX]{x03BC}\text{m}$ (Krishnan et al. Reference Krishnan, Fey, Stansfield, Landry, Nguy, Klassen and Robertson2012) in order to facilitate extended airborne duration. For this range of droplet sizes, it is likely that inertial effects are small, and the collision between these disinfectant aerosol droplets and virus-bearing droplet nuclei might be dominated by diffusion. It is unclear if the collision rate will be enhanced by the turbulence generated by heating, ventilation and air-conditioning systems. Given that fogging machines have been widely employed in particle image velocimetry (PIV), experiments would be particularly well poised to study these phenomena.
$10~\unicode[STIX]{x03BC}\text{m}$ (Krishnan et al. Reference Krishnan, Fey, Stansfield, Landry, Nguy, Klassen and Robertson2012) in order to facilitate extended airborne duration. For this range of droplet sizes, it is likely that inertial effects are small, and the collision between these disinfectant aerosol droplets and virus-bearing droplet nuclei might be dominated by diffusion. It is unclear if the collision rate will be enhanced by the turbulence generated by heating, ventilation and air-conditioning systems. Given that fogging machines have been widely employed in particle image velocimetry (PIV), experiments would be particularly well poised to study these phenomena.
4.3 Hand washing
Transmission of infection from surfaces with virion-laden respiratory droplets usually occurs via hands (Nicas & Jones Reference Nicas and Jones2009), and hand washing with soap therefore remains the most effective strategy for mitigating this mode of transmission (Stock & Francis Reference Stock and Francis1940). Soap molecules have a polar ionic hydrophilic side and a non-polar hydrophobic side that bonds with oils and lipids. Hand washing therefore works by emulsifying the lipid content of the material adhering to the hand into the bulk fluid and convecting it away. For enveloped viruses such as SARS-CoV-2, soap molecules also dismantle the lipid envelope of the virus, thereby deactivating it (Kohn, Gitelman & Inbar Reference Kohn, Gitelman and Inbar1980). The detritus from this disintegration is then trapped by the soap molecules into micelles, which are washed away.
These molecular-level mechanisms are powered by macro-level flow phenomena associated with the movements of the hands. Amazingly, despite the $170+$ year history of hand washing in medical hygiene (Rotter Reference Rotter1997), we were unable to find a single published research article on the flow physics of hand washing. The relative movement of the hands generates complex shear-driven flows of the soapy water, which forms a foam-laden, multiphase emulsion. Soap bubbles, which trap micelles, segregate rapidly from the fluid phase, thereby further accelerating the removal process.
$170+$ year history of hand washing in medical hygiene (Rotter Reference Rotter1997), we were unable to find a single published research article on the flow physics of hand washing. The relative movement of the hands generates complex shear-driven flows of the soapy water, which forms a foam-laden, multiphase emulsion. Soap bubbles, which trap micelles, segregate rapidly from the fluid phase, thereby further accelerating the removal process.
It is known that liquid foam exhibits elastic and plastic deformation under small and large stresses, respectively. With large enough deformation rates, the foam can rearrange its network and flow (Weaire & Hutzler Reference Weaire and Hutzler1999), which can be studied experimentally (Janiaud & Graner Reference Janiaud and Graner2005). Reynolds numbers for these soapy liquid layers could exceed $O(1000)$, suggesting that inertia, viscosity, surface tension and gravitational forces would all play an important role in this process. An improved understanding of hand-washing flow physics in the COVID-19 era could provide a science-based foundation for public guidelines/recommendations as well as new technologies that could improve the effectiveness of this practice.
$O(1000)$, suggesting that inertia, viscosity, surface tension and gravitational forces would all play an important role in this process. An improved understanding of hand-washing flow physics in the COVID-19 era could provide a science-based foundation for public guidelines/recommendations as well as new technologies that could improve the effectiveness of this practice.
4.4 Face masks
One issue that has generated significant controversy during the COVID-19 pandemic is the effectiveness of face masks (Dwyer & Aubrey Reference Dwyer and Aubrey2020; Elegant Reference Elegant2020; Feng et al. Reference Feng, Shen, Xia, Song, Fan and Cowling2020). Indeed, it is likely that the years ahead will see the use of face masks become a norm in our lives. Understanding the physics that underpins the effectiveness of face masks as a defence against airborne pathogens is, therefore, more important than ever.
Face masks provide ‘inward’ protection by filtering virus-laden aerosolized particles that would otherwise be inhaled by an uninfected person, and ‘outward’ protection by trapping virus-laden droplets expelled by an infected person (van der Sande, Teunis & Sabel Reference van der Sande, Teunis and Sabel2008). The effectiveness of a simple face mask such as the surgical, N95 or homemade cloth face mask is a function of the combined effect of the filtering properties of the face-mask material, the fit of the mask on the face and the related leaks from the perimeter of the face mask. Each of these features implicates complex flow phenomena, which are briefly addressed here.
4.4.1 Inward protection
The face-mask material traps droplets and particles via the combined effects of diffusion, inertial impaction, interception and electrostatic attraction (Thomas et al. Reference Thomas, Charvet, Bardin-Monnier and Appert-Collin2016; Fleming Reference Fleming2020). Filter efficiency (the ratio of the particle concentrations upstream and downstream of the mask) is a function of the particle- and fibre-size-based Reynolds numbers, fibre-based Péclet number (for diffusion), particle-to-fibre size ratio (for interception) and Stokes number (for impaction). The nonlinear variation of filtration mechanisms on these parameters generates a complex dependence of the filter efficiency on flow velocity, particle size and filter material characteristics such as pore size, fibre diameter and electrostatic charge.
The process of inhalation generates a low pressure in the region interior to the face mask, thereby sealing (or at least reducing) perimeter leaks. Thus, with a reasonably well-fitted face mask, inward protection depends primarily on the face-mask filter material. In this regard, an important characteristic of a face mask is the dependence of filtration efficiency on particle size. Studies (Chen & Willeke Reference Chen and Willeke1992; Weber et al. Reference Weber, Willeke, Marchloni, Myojo, Mckay, Donnelly and Liebhaber1993; Bałazy et al. Reference Bałazy, Toivola, Adhikari, Sivasubramani, Reponen and Grinshpun2006) have shown that, for a given filter, there is an intermediate particle size where filtration efficiency is minimum. Below this size, electrostatic attraction (active for masks such as N95) and diffusion dominate filtration, whereas above this size, impaction and interception are the dominant mechanisms. Aerosolized virus-laden droplets and droplet nuclei vary in size from submicrometre to millimetres, and therefore the aforementioned size-dependent filtration efficiency is an important consideration for inward protection against COVID-19 infections. An increase in fibre density to enhance filtration efficiency is accompanied by an increased pressure drop across the mask (Lai, Poon & Cheung Reference Lai, Poon and Cheung2012), which requires a greater inhalation effort by the wearer. Thus, an appropriate balance has to be achieved via proper design of the filter material, and this might be particularly important in the post-COVID-19 era, if wearing face masks becomes routine.
4.4.2 Outward protection
The outward protection afforded by face masks has emerged as a particularly important issue in the COVID-19 pandemic because a SARS-CoV-2 transmission may occur early in the course of infection, not only from symptomatic patients but also from asymptomatic as well as minimally symptomatic patients (Bai et al. Reference Bai, Yao, Wei, Tian, Jin, Chen and Wang2020; Ye et al. Reference Ye, Xu, Rong, Xu, Liu, Deng, Liu and Xu2020; Zou et al. Reference Zou, Ruan, Huang, Liang, Huang, Hong, Yu, Kang, Song and Xia2020). Indeed, the late switch to recommending universal use of face masks in the USA (Dwyer & Aubrey Reference Dwyer and Aubrey2020; Elegant Reference Elegant2020) was based on the recognition that this spread by asymptomatic hosts might be a significant driver of COVID-19 infections.
While a mask can significantly reduce the velocity of the throughflow jet during expiratory events (Tang et al. Reference Tang, Liebner, Craven and Settles2009), the increased pressure in the region between the mask and the face pushes the face mask outwards, resulting in increased perimeter leakage (Liu et al. Reference Liu, Lee, Mullins and Danisch1993; Lei et al. Reference Lei, Yang, Zhuang and Roberge2013). This fluid–structure interaction problem is mediated by the structural design as well as the permeability of the mask. The leakage jets that issue from the perimeter can be turbulent and highly directed (see, for example, the flow visualization in Tang et al. (Reference Tang, Liebner, Craven and Settles2009)), potentially serving as effective dispersers of respiratory aerosols in transverse directions. Spasmodic expiratory events such as coughing and sneezing that generate high transient expulsion velocities will significantly diminish the outward protection effectiveness of face masks (Lai et al. Reference Lai, Poon and Cheung2012). However, in a conceivable future where people will wear face masks while engaged in their daily routines, mask effectiveness during normal activities such as breathing and talking might be equally important.
In contrast to the problem of inward protection, which has been studied extensively (Chen & Willeke Reference Chen and Willeke1992; Weber et al. Reference Weber, Willeke, Marchloni, Myojo, Mckay, Donnelly and Liebhaber1993; Bałazy et al. Reference Bałazy, Toivola, Adhikari, Sivasubramani, Reponen and Grinshpun2006), the flow physics of outward protection from face masks is less well studied. Tang et al. (Reference Tang, Liebner, Craven and Settles2009) used Schlieren imaging to visualize cough-induced flow with and without face masks (surgical and N95). The study was extremely inventive but mostly qualitative, and future experiments should provide quantitative analysis of the leakage and throughflow jets, the aerosol dispersion through these jets, as well as the deformation of the mask during a variety of expiratory events. Recent CFD studies of face-mask aerodynamics (Lei et al. Reference Lei, Yang, Zhuang and Roberge2013; Zhu et al. Reference Zhu, Lee, Wang and Lee2016) demonstrate the potential of computational modelling for this problem, but there is a critical need for modelling flow-induced billowing and associated leakage enhancement during expiratory events. Ultimately, analysis should not only enable a detailed evaluation of the protective efficiency of face masks; it should also drive design changes that enhance mask performance and provide data that inform guidelines on practices such as social distancing.
5 Closing
The COVID-19 pandemic has exposed significant scientific gaps in our understanding of critical issues, ranging from the transmission pathways of such respiratory diseases, to the strategies to use for mitigating these transmissions. This article summarizes a fluid dynamicist’s perspective on important aspects of the problem, including respiratory droplet formation, two-phase expiratory flows, droplet evaporation and transport, and face-mask aerodynamics. COVID-19 touches almost every major arena of fluid dynamics, from hydrodynamic instability to porous-media and turbulent shear flows, from droplet breakup to particle deposition, and from Newtonian gas flows to non-Newtonian liquids. For the topics that we have discussed, breadth and epidemiological context have taken precedence over a detailed exposition. COVID-19 has thrust the field of fluid dynamics into the public eye in a way (Bourouiba Reference Bourouiba2020; Parshina-Kottas et al. Reference Parshina-Kottas, Saget, Patanjali, Fleisher and Gianordoli2020) not seen since the space race of the 1960s. Our hope is that not only will this article serve as a call-to-arms to fluid dynamicists, it will also provide a starting point for the researcher who is motivated to tackle the science of COVID-19, and other similar diseases that are sure to appear in the not-too-distant future.
Acknowledgements
We gratefully acknowledge A. Berdia, and Drs C. McQueen, A. Prosperetti, H. S. Udaykumar, G. Settles and C. Machamer, who reviewed and commented on drafts of the manuscript.
Declaration of interests
The authors report no conflict of interests.