Article contents
Nonlinear dynamics of two-layer channel flow with soluble surfactant below or above the critical micelle concentration
Published online by Cambridge University Press: 04 August 2020
Abstract
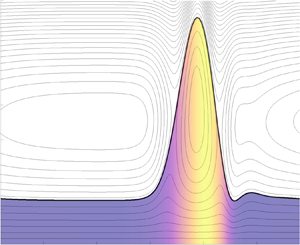
The nonlinear stability of an inertialess two-layer surfactant-laden Couette flow is considered. The two fluids are immiscible and have different thicknesses, viscosities and densities. One of the fluids is contaminated with a soluble surfactant whose concentration may be above the critical micelle concentration, in which case micelles are formed in the bulk of the fluid. A surfactant kinetic model is adopted that includes the adsorption and desorption of molecules to and from the interface, and the formation and breakup of micelles in the bulk. The lubrication approximation is applied and a strongly nonlinear system of equations is derived for the evolution of the interface and surfactant concentration at the interface, as well as the vertically averaged monomer and micelle concentrations in the bulk (as a result of fast vertical diffusion). The primary aim of this study is to determine the influence of surfactant solubility on the nonlinear dynamics. The nonlinear lubrication model is solved numerically in periodic domains and saturated travelling waves are obtained at large times. It is found that a sufficiently soluble surfactant can either destabilise or stabilise the interface depending on certain fluid properties. The stability behaviour of the system depends crucially on the values of the fluid viscosity ratio  $m$ and thickness ratio
$m$ and thickness ratio  $n$ in reference to the boundary
$n$ in reference to the boundary  $m=n^{2}$. If the surfactant exists at large concentrations that exceed the critical micelle concentration, then long waves are stable at large times, unless density stratification effects overcome the stabilising influence of micelles. Travelling wave bifurcation branches are also calculated and the impact of various parameters (such as the domain length or fluid thickness ratio) on the wave shapes, amplitudes and speeds is examined. The mechanism responsible for interfacial (in)stability is explained in terms of the phase difference between the interface deformation and concentration waves, which is shifted according to the sign of the crucial factor
$m=n^{2}$. If the surfactant exists at large concentrations that exceed the critical micelle concentration, then long waves are stable at large times, unless density stratification effects overcome the stabilising influence of micelles. Travelling wave bifurcation branches are also calculated and the impact of various parameters (such as the domain length or fluid thickness ratio) on the wave shapes, amplitudes and speeds is examined. The mechanism responsible for interfacial (in)stability is explained in terms of the phase difference between the interface deformation and concentration waves, which is shifted according to the sign of the crucial factor  $(m-n^{2})$ and the strength of the surfactant solubility.
$(m-n^{2})$ and the strength of the surfactant solubility.
Information
- Type
- JFM Papers
- Information
- Copyright
- © The Author(s), 2020. Published by Cambridge University Press
References
REFERENCES
Kalogirou and Blyth supplementary movie 1
Time evolution of the interfacial wave (top), interfacial surfactant concentration (middle), and solution norm (bottom) for h0 = 0:4, m = 0:5, = 0:5 and Kb ≫ 1. This movie, together with Movies 2 and 3, demonstrate stabilisation of the interface for a sufficiently strong surfactant solubility.
Kalogirou and Blyth supplementary movie 2
Time evolution of the interfacial wave (top), interfacial surfactant concentration (middle), and solution norm (bottom) for h0 = 0:4, m = 0:5, = 0:5 and Kb = 5. This movie, together with Movies 1 and 3, demonstrate stabilisation of the interface for a sufficiently strong surfactant solubility.
Kalogirou and Blyth supplementary movie 3
Time evolution of the interfacial wave (top), interfacial surfactant concentration (middle), and solution norm (bottom) for h0 = 0:4, m = 0:5, = 0:5 and Kb = 2. This movie, together with Movies 1 and 2, demonstrate stabilisation of the interface for a sufficiently strong surfactant solubility.
Kalogirou and Blyth supplementary movie 4
Time evolution of the interfacial wave (top), interfacial surfactant concentration (middle), and solution norm (bottom) for h0 = 0:4, m = 5, = 0:5 and Kb ≫ 1. This movie, together with Movie 5, demonstrate destabilisation of the interface for a sufficiently strong surfactant solubility.
Kalogirou and Blyth supplementary movie 5
Time evolution of the interfacial wave (top), interfacial surfactant concentration (middle), and solution norm (bottom) for h0 = 0:4, m = 5, = 0:5 and Kb = 2. This movie, together with Movie 4, demonstrate destabilisation of the interface for a sufficiently strong surfactant solubility.
Kalogirou and Blyth supplementary movie 6
Time evolution of the interfacial wave (top), interfacial surfactant concentration (middle), and solution norm (bottom) for h0 = 0:2, m = 0:5, = 0:75 and Kb ≫ 1. This movie, together with Movie 7, demonstrate stabilisation of the interface for bulk concentrations above the critical micelle concentration.
Kalogirou and Blyth supplementary movie 7
Time evolution of the interfacial wave (top), interfacial surfactant concentration (middle), and solution norm (bottom) for h0 = 0:2, m = 0:5, = 0:75 and Kb = 3. This movie, together with Movie 6, demonstrate stabilisation of the interface for bulk concentrations above the critical micelle concentration.
- 7
- Cited by

