Article contents
On the dispersion of a drug delivered intrathecally in the spinal canal
Published online by Cambridge University Press: 27 December 2018
Abstract
This paper investigates the transport of a solute carried by the cerebrospinal fluid (CSF) in the spinal canal. The analysis is motivated by the need for a better understanding of drug dispersion in connection with intrathecal drug delivery (ITDD), a medical procedure used for treatment of some cancers, infections and pain, involving the delivery of the drug to the central nervous system by direct injection into the CSF via the lumbar route. The description accounts for the CSF motion in the spinal canal, described in our recent publication (Sánchez et al., J. Fluid Mech., vol. 841, 2018, pp. 203–227). The Eulerian velocity field includes an oscillatory component with angular frequency 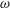 $\unicode[STIX]{x1D714}$, equal to that of the cardiac cycle, and associated tidal volumes that are a factor
$\unicode[STIX]{x1D714}$, equal to that of the cardiac cycle, and associated tidal volumes that are a factor 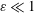 $\unicode[STIX]{x1D700}\ll 1$ smaller than the total CSF volume in the spinal canal, with the small velocity corrections resulting from convective acceleration providing a steady-streaming component with characteristic residence times of order
$\unicode[STIX]{x1D700}\ll 1$ smaller than the total CSF volume in the spinal canal, with the small velocity corrections resulting from convective acceleration providing a steady-streaming component with characteristic residence times of order 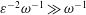 $\unicode[STIX]{x1D700}^{-2}\unicode[STIX]{x1D714}^{-1}\gg \unicode[STIX]{x1D714}^{-1}$. An asymptotic analysis for
$\unicode[STIX]{x1D700}^{-2}\unicode[STIX]{x1D714}^{-1}\gg \unicode[STIX]{x1D714}^{-1}$. An asymptotic analysis for 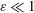 $\unicode[STIX]{x1D700}\ll 1$ accounting for the two time scales
$\unicode[STIX]{x1D700}\ll 1$ accounting for the two time scales 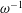 $\unicode[STIX]{x1D714}^{-1}$ and
$\unicode[STIX]{x1D714}^{-1}$ and 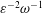 $\unicode[STIX]{x1D700}^{-2}\unicode[STIX]{x1D714}^{-1}$ is used to investigate the prevailing drug-dispersion mechanisms and their dependence on the solute diffusivity, measured by the Schmidt number
$\unicode[STIX]{x1D700}^{-2}\unicode[STIX]{x1D714}^{-1}$ is used to investigate the prevailing drug-dispersion mechanisms and their dependence on the solute diffusivity, measured by the Schmidt number 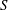 $S$. Convective transport driven by the time-averaged Lagrangian velocity, obtained as the sum of the Eulerian steady-streaming velocity and the Stokes-drift velocity associated with the non-uniform pulsating flow, is found to be important for all values of
$S$. Convective transport driven by the time-averaged Lagrangian velocity, obtained as the sum of the Eulerian steady-streaming velocity and the Stokes-drift velocity associated with the non-uniform pulsating flow, is found to be important for all values of  $S$. By way of contrast, shear-enhanced Taylor dispersion, which is important for values of
$S$. By way of contrast, shear-enhanced Taylor dispersion, which is important for values of 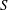 $S$ of order unity, is shown to be negligibly small for the large values
$S$ of order unity, is shown to be negligibly small for the large values 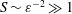 $S\sim \unicode[STIX]{x1D700}^{-2}\gg 1$ corresponding to the molecular diffusivities of all ITDD drugs. Results for a model geometry indicate that a simplified equation derived in the intermediate limit
$S\sim \unicode[STIX]{x1D700}^{-2}\gg 1$ corresponding to the molecular diffusivities of all ITDD drugs. Results for a model geometry indicate that a simplified equation derived in the intermediate limit  $1\ll S\ll \unicode[STIX]{x1D700}^{-2}$ provides sufficient accuracy under most conditions, and therefore could constitute an attractive reduced model for future quantitative analyses of drug dispersion in the spinal canal. The results can be used to quantify dependences of the drug-dispersion rate on the frequency and amplitude of the pulsation of the intracranial pressure, the compliance and specific geometry of the spinal canal and the molecular diffusivity of the drug.
$1\ll S\ll \unicode[STIX]{x1D700}^{-2}$ provides sufficient accuracy under most conditions, and therefore could constitute an attractive reduced model for future quantitative analyses of drug dispersion in the spinal canal. The results can be used to quantify dependences of the drug-dispersion rate on the frequency and amplitude of the pulsation of the intracranial pressure, the compliance and specific geometry of the spinal canal and the molecular diffusivity of the drug.
- Type
- JFM Papers
- Information
- Copyright
- © 2018 Cambridge University Press
References
- 23
- Cited by


