Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by
Crossref.
Lee, Jae H.
Rygg, Alex D.
Kolahdouz, Ebrahim M.
Rossi, Simone
Retta, Stephen M.
Duraiswamy, Nandini
Scotten, Lawrence N.
Craven, Brent A.
and
Griffith, Boyce E.
2020.
Fluid–Structure Interaction Models of Bioprosthetic Heart Valve Dynamics in an Experimental Pulse Duplicator.
Annals of Biomedical Engineering,
Vol. 48,
Issue. 5,
p.
1475.
Lee, Jae H.
Scotten, Lawrence N.
Hunt, Robert
Caranasos, Thomas G.
Vavalle, John P.
and
Griffith, Boyce E.
2021.
Bioprosthetic aortic valve diameter and thickness are directly related to leaflet fluttering: Results from a combined experimental and computational modeling study.
JTCVS Open,
Vol. 6,
Issue. ,
p.
60.
Soltany Sadrabadi, Mohammadreza
Hedayat, Mohammadali
Borazjani, Iman
and
Arzani, Amirhossein
2021.
Fluid-structure coupled biotransport processes in aortic valve disease.
Journal of Biomechanics,
Vol. 117,
Issue. ,
p.
110239.
Chen, Ye
Lu, Xiao
Luo, Haoxiang
and
Kassab, Ghassan S.
2022.
Aortic Leaflet Stresses Are Substantially Lower Using Pulmonary Visceral Pleura Than Pericardial Tissue.
Frontiers in Bioengineering and Biotechnology,
Vol. 10,
Issue. ,
Johnson, Emily L.
Rajanna, Manoj R.
Yang, Cheng-Hau
and
Hsu, Ming-Chen
2022.
Effects of membrane and flexural stiffnesses on aortic valve dynamics: Identifying the mechanics of leaflet flutter in thinner biological tissues.
Forces in Mechanics,
Vol. 6,
Issue. ,
p.
100053.
Vogl, Brennan J.
Niemi, Nicholas R.
Griffiths, Leigh G.
Alkhouli, Mohamad A.
and
Hatoum, Hoda
2022.
Impact of calcific aortic valve disease on valve mechanics.
Biomechanics and Modeling in Mechanobiology,
Vol. 21,
Issue. 1,
p.
55.
Poon, Eric K. W.
Ono, Masafumi
Wu, Xinlei
Dijkstra, Jouke
Sato, Yu
Kutyna, Matthew
Torii, Ryo
Reiber, Johan H. C.
Bourantas, Christos V.
Barlis, Peter
El-Kurdi, Mohammed S.
Cox, Martijn
Virmani, Renu
Onuma, Yoshinobu
and
Serruys, Patrick W.
2023.
An optical coherence tomography and endothelial shear stress study of a novel bioresorbable bypass graft.
Scientific Reports,
Vol. 13,
Issue. 1,
Bahadormanesh, Nikrouz
Tomka, Benjamin
Abdelkhalek, Mohamed
Khodaei, Seyedvahid
Maftoon, Nima
and
Keshavarz-Motamed, Zahra
2023.
A Doppler-exclusive non-invasive computational diagnostic framework for personalized transcatheter aortic valve replacement.
Scientific Reports,
Vol. 13,
Issue. 1,
Asadi, Hossein
and
Borazjani, Iman
2023.
A contact model based on the coefficient of restitution for simulations of bio‐prosthetic heart valves.
International Journal for Numerical Methods in Biomedical Engineering,
Vol. 39,
Issue. 9,
Tao, Li
Jingyuan, Zhou
Hongjun, Zhou
Yijing, Li
Yan, Xiong
and
Yu, Chen
2023.
Research on fatigue optimization simulation of polymeric heart valve based on the iterative sub‐regional thickened method.
International Journal for Numerical Methods in Biomedical Engineering,
Vol. 39,
Issue. 10,
Bornemann, Karoline-Marie
and
Obrist, Dominik
2024.
Instability mechanisms initiating laminar–turbulent transition past bioprosthetic aortic valves.
Journal of Fluid Mechanics,
Vol. 985,
Issue. ,
El-Nashar, Hussam
Sabry, Malak
Tseng, Yuan-Tsan
Francis, Nadine
Latif, Najma
Parker, Kim H.
Moore, James E.
and
Yacoub, Magdi H.
2024.
Multiscale structure and function of the aortic valve apparatus.
Physiological Reviews,
Vol. 104,
Issue. 4,
p.
1487.
Costa, Matheus Carvalho Barbosa
Gonçalves, Saulo de Freitas
Fleury, João Victor Curado
Silva, Mário Luis Ferreira da
Huebner, Rudolf
and
Avelar, Artur Henrique de Freitas
2024.
Computational fluid–structure analysis of the impact of leaflet thickness and protrusion height on the flutter phenomenon in aortic valve bioprostheses.
Meccanica,
Vol. 59,
Issue. 5,
p.
685.
Shu, Peng
Li, Daochun
Zhao, Shiwei
and
Lv, Rui
2024.
Effects of body posture on aortic valve hemodynamics and biomechanics using the fluid-structure interaction method.
Journal of Biomechanics,
Vol. 177,
Issue. ,
p.
112388.
Hashemifard, Alireza
Fatouraee, Nasser
and
Nabaei, Malikeh
2024.
Nature of aortic annulus: Influence of annulus dynamic on the aortic valve hemodynamics.
Computers in Biology and Medicine,
Vol. 181,
Issue. ,
p.
109037.
Abbas, Syed Samar
Asadi, Hossein
and
Borazjani, Iman
2025.
Closure dynamics of aortic mechanical heart valves versus bioprosthetic heart valves.
Journal of Fluid Mechanics,
Vol. 1012,
Issue. ,
Bornemann, Karoline-Marie
and
Obrist, Dominik
2025.
Leaflet fluttering changes laminar–turbulent transition mechanisms past bioprosthetic aortic valves.
Physics of Fluids,
Vol. 37,
Issue. 5,
Ertas, Atila
Farley-Talamantes, Erik
Cuvalci, Olkan
and
Gecgel, Ozhan
2025.
3D-Printing of Artificial Aortic Heart Valve Using UV-Cured Silicone: Design and Performance Analysis.
Bioengineering,
Vol. 12,
Issue. 1,
p.
94.
Shu, Peng
Li, Daochun
Lv, Rui
Li, Yongkang
Zhao, Shiwei
and
Xiang, Jinwu
2025.
Modulatory role of coronary flow in aortic valve hemodynamics: A fluid–structure interaction study.
Physics of Fluids,
Vol. 37,
Issue. 9,
Costa, Matheus Carvalho Barbosa
Gonçalves, Saulo de Freitas
Fleury, João Victor Curado
da Silva, Mário Luis Ferreira
Huebner, Rudolf
and
Avelar, Artur Henrique de Freitas
2025.
Comparison between the fluid–structure interaction approach and the finite element method approach to analyze the leaflet flutter in bioprosthetic aortic valve.
Journal of Biomechanics,
Vol. 181,
Issue. ,
p.
112532.
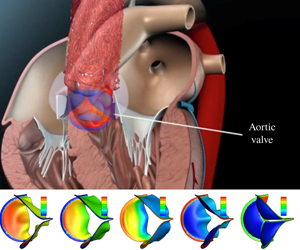
 $1.5\times 10^{-4}$ to 0.6. The non-uniform pressure distribution over the leaflets and the transient valve force are calculated, including the ‘water hammer’ effect during rapid closure. With low bending rigidity, the valve functions normally and produces physiological characteristics of healthy valves. However, exceedingly low rigidity leads to flapping motion of the leaflets and, in some cases (e.g. the normalized bending rigidity around 0.001), may reduce the performance index. As the leaflets become much stiffer, the valve is more difficult to open and slower to close, which leads to higher resistance and a reduced flow rate. Therefore, our results suggest that there is an optimal range of bending rigidity for the valve, roughly between 0.003 and 0.04 in normalized term. We further develop a one-dimensional unsteady flow model based on the momentum and mass conservation equations to replace the 3-D flow in the FSI simulation. The new flow model incorporates pressure loss across the valve as well as the leaflet motion. Comparison with the 3-D results shows that the reduced flow model is able to produce a reasonable 3-D deformation sequence of the leaflets, opening area and flow rate, especially in the cases of low bending rigidity.
$1.5\times 10^{-4}$ to 0.6. The non-uniform pressure distribution over the leaflets and the transient valve force are calculated, including the ‘water hammer’ effect during rapid closure. With low bending rigidity, the valve functions normally and produces physiological characteristics of healthy valves. However, exceedingly low rigidity leads to flapping motion of the leaflets and, in some cases (e.g. the normalized bending rigidity around 0.001), may reduce the performance index. As the leaflets become much stiffer, the valve is more difficult to open and slower to close, which leads to higher resistance and a reduced flow rate. Therefore, our results suggest that there is an optimal range of bending rigidity for the valve, roughly between 0.003 and 0.04 in normalized term. We further develop a one-dimensional unsteady flow model based on the momentum and mass conservation equations to replace the 3-D flow in the FSI simulation. The new flow model incorporates pressure loss across the valve as well as the leaflet motion. Comparison with the 3-D results shows that the reduced flow model is able to produce a reasonable 3-D deformation sequence of the leaflets, opening area and flow rate, especially in the cases of low bending rigidity.
