Article contents
Two-dimensional hydrodynamics of a Janus particle vesicle
Published online by Cambridge University Press: 04 May 2022
Abstract
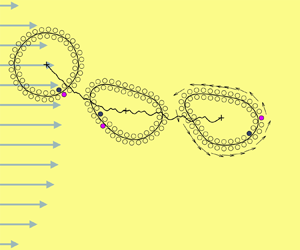
We develop a new model, to our knowledge, for the many-body hydrodynamics of amphiphilic Janus particles suspended in a viscous background flow. The Janus particles interact through a hydrophobic attraction potential that leads to self-assembly into bilayer structures. We adopt an efficient integral equation method for solving the screened Laplace equation for hydrophobic attraction and for solving the mobility problem for hydrodynamic interactions. The integral equation formulation accurately captures both interactions for near touched boundaries. Under a linear shear flow, we observe the tank-treading deformation in a two-dimensional vesicle made of Janus particles. The results yield measurements of intermonolayer friction, membrane permeability and, at large shear rates, membrane rupture. The simulation studies include a Janus particle vesicle in both linear and parabolic shear flows, and interactions between two Janus particle vesicles in shear and extensional flows. The hydrodynamics of the Janus particle vesicle is similar to the behaviour of an inextensible, elastic vesicle membrane with permeability.
Information
- Type
- JFM Papers
- Information
- Copyright
- © The Author(s), 2022. Published by Cambridge University Press
References
REFERENCES
Fu et al. Supplementary Movie 1
There are 58 circular particles with radius 1.25 nm that form a self-enclosed bilayer structure. The simulation result shows the vesicle relaxation and the fluid pressure inside the JP vesicle decreases to 0 after a period of time. The color field from blue to red shows the magnitude of fluid pressure and the range from 0 to 0.1 pN nm-2. All dark blue dots in the domain are tracers that move with the calculated fluid motion. This movie includes the simulation results for 1 μs.
Fu et al. Supplementary Movie 2
We adopt the relaxed configuration and place the JP vesicle in the shear flow. The simulation result shows a tank-treading motion for the shear rate χ=0.005. The colored field from dark blue to dark red shows the hydrophobic attraction activity and the range is from 0 to 1. All white dots in the domain are tracers in fluid that move with the fluid motion. This movie includes the simulation results for 4 μs.
Fu et al. Supplementary Movie 3
Using the same setup demonstrated in Movie S2, we show the simulation result for inter-monolayer slip by tracking a pair of particles (blue and yellow). After t=4000, the yellow particle in the outer leaflet surpasses the blue particle in the inner leaflet by the distance about 1 particle diameter. This movie includes the simulation results for 4 μs.
Fu et al. Supplementary Movie 4
The numerical results show that the JP vesicle ruptures at the shear rate χ=0.0655. The colored field from dark blue to dark red shows the hydrophobic attraction activity and the range is from 0 to 1. All white dots in the domain are tracers in fluid that move with the fluid motion. This movie includes the simulation results for 0.6 μs.
Fu et al. Supplementary Movie 5
There are two relaxed JP vesicles suspended in a shear flow. The two centroids are at coordinates (-25,0) and (25,0) in nm. The colored field from dark blue to dark red shows the hydrophobic attraction activity and the range is from 0 to 1. All white dots in the domain are tracers in fluid that move with the fluid motion. This movie includes the simulation results for 4 μs.
Fu et al. Supplementary Movie 6
There are two relaxed JP vesicles suspended in an extensional flow. The centroid of the left JP vesicle is 0.25 nm above the x-axis and the right JP vesicle is 0.25 nm below the x-axis. The colored field from dark blue to dark red shows the hydrophobic attraction activity and the range is from 0 to 1. All white dots in the domain are tracers in fluid that move with the fluid motion. This movie includes the simulation results for 1.2 μs.
- 2
- Cited by


