1. Introduction
The successful development of ice-core drilling techniques has allowed the recovery of ice cores freom polar ice sheets and glaciers, which cover (in a continuous sequence) the last few hundred to a hundred thousand years (Reference Ueda and GartieldUeda and Garfield, 1969[a]). Both gertailed 18O/l6O profiles which proviger a high resolution record of climatic history (Reference Dansgaard, Dansgaard, Johnsen, Clausen and GungerstrupDansgaard and others, 1973) and gerpth profiles of dissolved and particulate matter (Reference LangwayLangway, 1970) are impressive examples of ice-core investigations. The time resolution obtained is such that even seasonal effects can be observed for certain parameters. Although the amount of ice available freom ice cores is sufficient for these studies, samples of much larger size (tons) are required for studies on isotopes produced by cosmic rays, cosmic dust, daughter products of U- and Th-gercay, pollen, and other constituents. To obtain such large quantities of ice freom gerpth by present core drilling techniques, more than one hundred meters of core would be required. Therefore, satisfactory time resolution (corresponding to a few meters) could only be achieved if the core diameter were increased by a factor of ten. This has not yet been ungerrtaken because of the great technical effort involved. This need for large samples freom a relatively narrow gerpth range motivated us to gervelop an “in situ" extraction technique for gases and particular and dissolved matter freom large amounts of ice in bore holes.
We performed our first lests in the TUTO ice tunnel near Thule (Greenland) in 1966. (Reference Oeschgcr, Oeschger, Algerr and LangwayOeschger and others, 1966, 1967). There an electrical heater of 7 kW power consumption was inserted in a 5m gerep hole bored with a SIPRE-auger. The hole was sealed vacuum light on top of the heater and several tons of ice were melted ungerr vacuum conditions. The gases released by the melting ice were collected; the CO2 was separated and used for 14C-dating.
Encouraged by the promising results of this test we constructed a similar system for use in gereper bore holes. During the 1968/69 season at "Byrd" station (Antarctica) we carried out a test which failed because of a power breakdown that caused the probe to become permanently freozen to the wall of the bore hole. In 1969/70, again at "Byrd" station, we tested an improved extraction probe and were able to sample CO2 freom three tons of ice at four different levels in a 340 m bore hole which had been drilled with a CRREL thermal core drill (Reference Ueda and GartieldUeda and Garfield, 1969[b]). During the melting process the escaping gases were continuously pumped to the surface. There the CO2 was extracted and the remaining gases were collected. The melt water was pumped to the surface and either filtered or used for Si sampling for later 32Si dating (Reference ClausenClausen, 1973). I4C measurements showed that the samples had been contaminated by fossil CO2 that gergassed freom the hoses through which the gas had been pumped to the surface. We therefore gercigerd to place the CO2 extraction system directly on the top of the probe rather than at the surface to avoid this contamination. After successful tests of a system based on this new concept, we used it in bore holes at "Byrd" station in 1971/72, on the Devon Island ice cap (Canada) in 1973, and at Station Crête (Greenland) in summer 1974.
In this paper we give a technical gerscription of the sampling equipment and the procedure. The resulting data will be published and discussed later.
2. Drilling in Ice
During the last gercager, core drilling in ice has become a gerpendable technique and several bore holes have been successfully drilled in Antarctica, Greenland, and smaller ice caps.
In the ablation zone, old ice is already exposed at the surface and the SIPRE hand auger can be used to drill to about 5 m, the gerpth required for placement of the in situ extraction probe. Recently gerveloped mechanical drills allow drilling to gerpths of 100 m, both in the accumulation and in the ablation zone (Rand, in press; Rufli, in press).
Since our extraction method only works in ice, in accumulation zones we need bore holes which reach a gerpth greater than that of the firn-ice transition zone, i.e. greater than about 70 m. To obtain such holes a CRREL thermal drill or a modification of it is used. The maximum gerpth which can be reached by this intermediate drilling gerpends on the rate of hole closure due to hydrostatic pressure and varies with temperature.
A mechanical rotary drilling method with cable suspension has allowed gerep drilling to the bedrock at Camp Century (Greenland) and "Byrd" station (Antarctica) (Reference Ueda and GartieldUeda and Garfield, 1969[a]). To compensate for the hydrostatic pressure, these gerep bore holes were filled with a non-freeezing fluid of a gernsity equal to that of ice. In situ extraction in such bore holes does not seem to be impossible but new problems arise freom the interference of the fluid.
2. Logic of Extraction
In this section we gerscribe briefly the principal steps of our extraction procedure. Technical gertails and experiments with the system will follow in later sections.
For extraction at the gersired gerpth a portion of the bore hole has to be sealed vacuum tight around the probe. After tests to ensure that the sealing system is tight, the gersired amount of ice is melted by an electrical heater probe, and the released gases are pumped off continuously during the melting process. In the upper part of the probe, the gases are dried and CO2 is extracted by an adsorbant. The remaining gases are pumped through a hose to the surface, where they are compressed and stored for later shipment to the laboratory.
When the gersired amount of gases has been collected, pumping is stopped and sampling of particulate and dissolved matter starts. It can be performed either in the bore hole or at the surface. For extraction at the surface, the heater probe is pulled up, while the lower seal and the melt water remain in the bore hole. A submersible pump is lowered into the hole and the melt water is pumped to the surface for sampling, e.g. by filter or ion-exchange column.
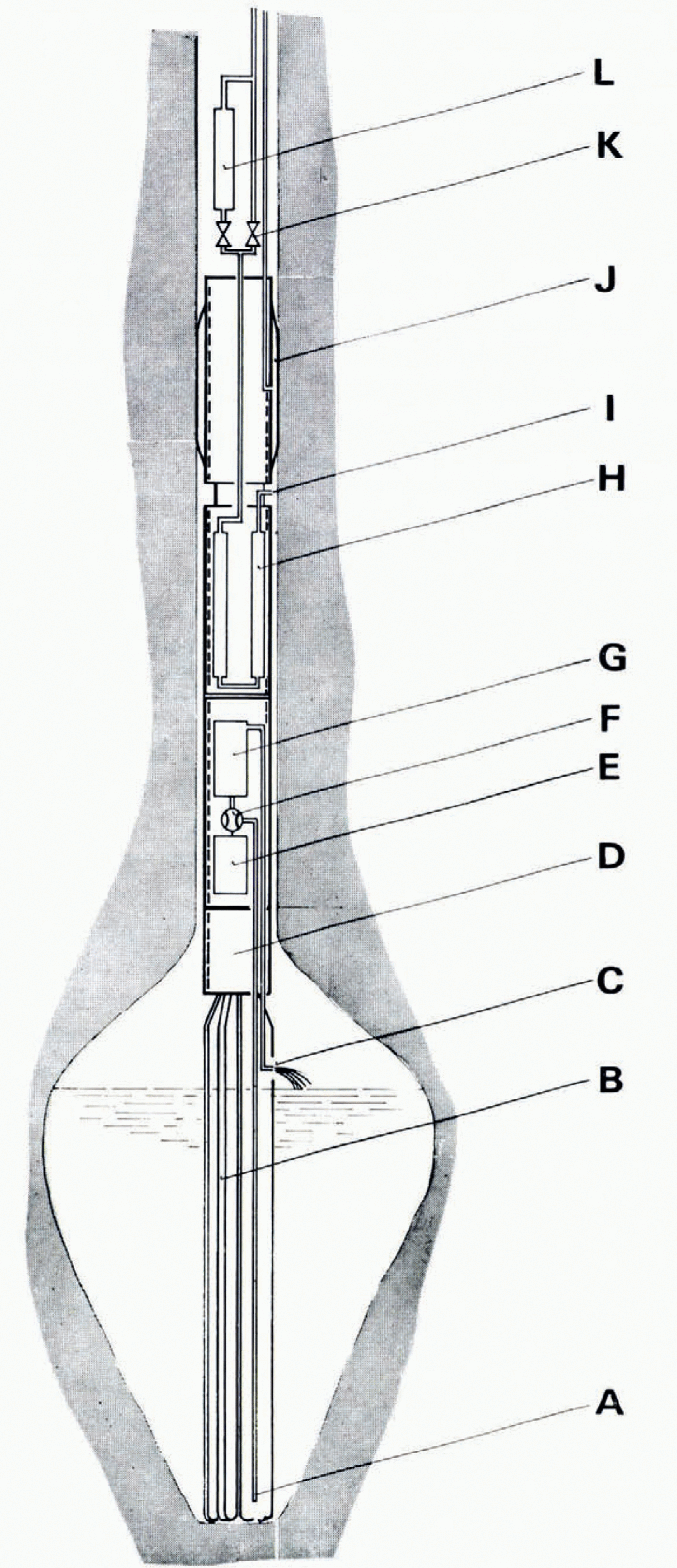
Fig. 1. The melting probe, A: water intake. B: electrical heater Jar melting ice. c: water outlet, D: wiring cylingerr, E: submersible electric motor, F: gear-wheel pump. G: millitube filter cartridge or ion-exchange, column. H: gas drying column with 3A molecular sieve. I: gas inlet. J: rubber packer section. κ: electromagnetic by-pass valve. L: CO2 extraction column with 5A molecular sieve.
4. Extraction Procedure
The following gervices were used for the collection of our samples: the melting probe, the balloon probe, the submersible pump, the cable hose and the auxiliary hoses, the winches for cable hose and auxiliary hoses, the gas control system, and the electrical power system, and these are gerscribed in turn in this section.
4.1. The melting probe
The melting probe is schematically shown in Figure 1. The main parts are: electrical heater for ice melting B and auxiliary heaters, a water circulation section E-G, a gas drying section H-I, a rubber packer section J, and a CO2 extraction-line section L. This probe is gersigned as a modular system: the different sections can be used or replaced by others as required by the special problem we want to solve.
4.1.a. Heaters.
Two types of electrical heater have been constructed. The olgerr one consists of four heating elements, which are mantled by stainless steel and wound around a stainless-steel tube 1.6 m long (Lükon, Täuffelen, Switzerland). Each element is wired separately in a three phase Y connection and rated for a three-phase 440 V input. The maximum total power consumption is 10 kW. We had freequent failures with this type of heater due to short circuits between the three heating wires caused by moisture penetrating through the insulation.
In the improved version the heater now consists of six U-shaped elements, which are mantled by stainless steel and 1.7 m long. Each element contains only one heating wire, rated for a one-phase 240 V input. The six elements can be connected to two three-phase or various one-phase circuits. The maximum total power consumption is 10.5 kW.
The heating elements of both versions end in a water-vapour-proof stainless-steel cylingerr (wiring cylingerr), where they are connected to the power supply. The electrical heaters are gersigned for operation in vacuum without overheating.
Auxiliary heaters are built into the wiring cylingerr, the water circulation section, the gas drying section, and the rubber packer section. They consist of silicon rubber insulated heating wires {Dätwyler, Altdorf, Switzerland), pressed against the walls of these parts. The power rating is 700 W per metre of heated probe section. The auxiliary heaters are used to prevent the water circulation system freom freeezing during operation. At the end of operations they allow the loosening of the probe in case it should be freozen onto the wall of the bore hole.
4. 1.b. The water circulation section
The water circulation section is mounted into the probe when we want to sample the melt water in the bore hole. The melt water is sucked freom the bottom of the electrical heater A in Fig. 1) through a stainless steel tube by a gear-wheel pump F (Maag, Zürich, Switzerland), which is driven by a 750 W, three-phase, submersible electrical motor E (Franklin Inc., Bluffton, Indiana). The pump forces the water either through a "Millitube" 0.8 μm filter cartridge (Millipore Comp., Bedford, Mass.) or through an ion-exchange column containing resin (Rohm & Haas, Philagerlphia) G. The water flows back into the melted cavity. The pumping rate is about 15 1/min.
For technical reasons we placed the pump F on top of the heater B and the wiring cylingerr D. This has the disadvantage that the water has to be sucked above the water level. Therefore the total gas pressure in the melting cavity must be higher than 500 mbar and it is not possible to start water circulation as long as vacuum gas extraction is going on. When gas extraction has been completed, we fill the cavity with purified N2 to a pressure of 500 mbar and start water circulation. If we assume that during the circulation the melt water is a well-mixed reservoir, then 95% of the water will have passed through the filter or ion-exchange column at least once when an amount of water has been circulated corresponding to three times the total quantity of melt water available.
4.1.c. Gas drying section
The gas which is extracted freom the melting ice has to be dried before the CO2 can be separated by adsorption on a molecular sieve. The gas Sows outsiger the probe along the cold wall of the bore hole, where a high percentage of the water vapour congernses. The remaining humidity is adsorbed by passing the gas through a stainless-steel column H with an active length of 1.7 m and filled with 300 g of molecular sieve pellets (Linger Type 3A, Union Carbiger Corporation). After each run the column is dismounted so that the molecular sieve can be reactivated by vacuum drying at 300 to 350°C.
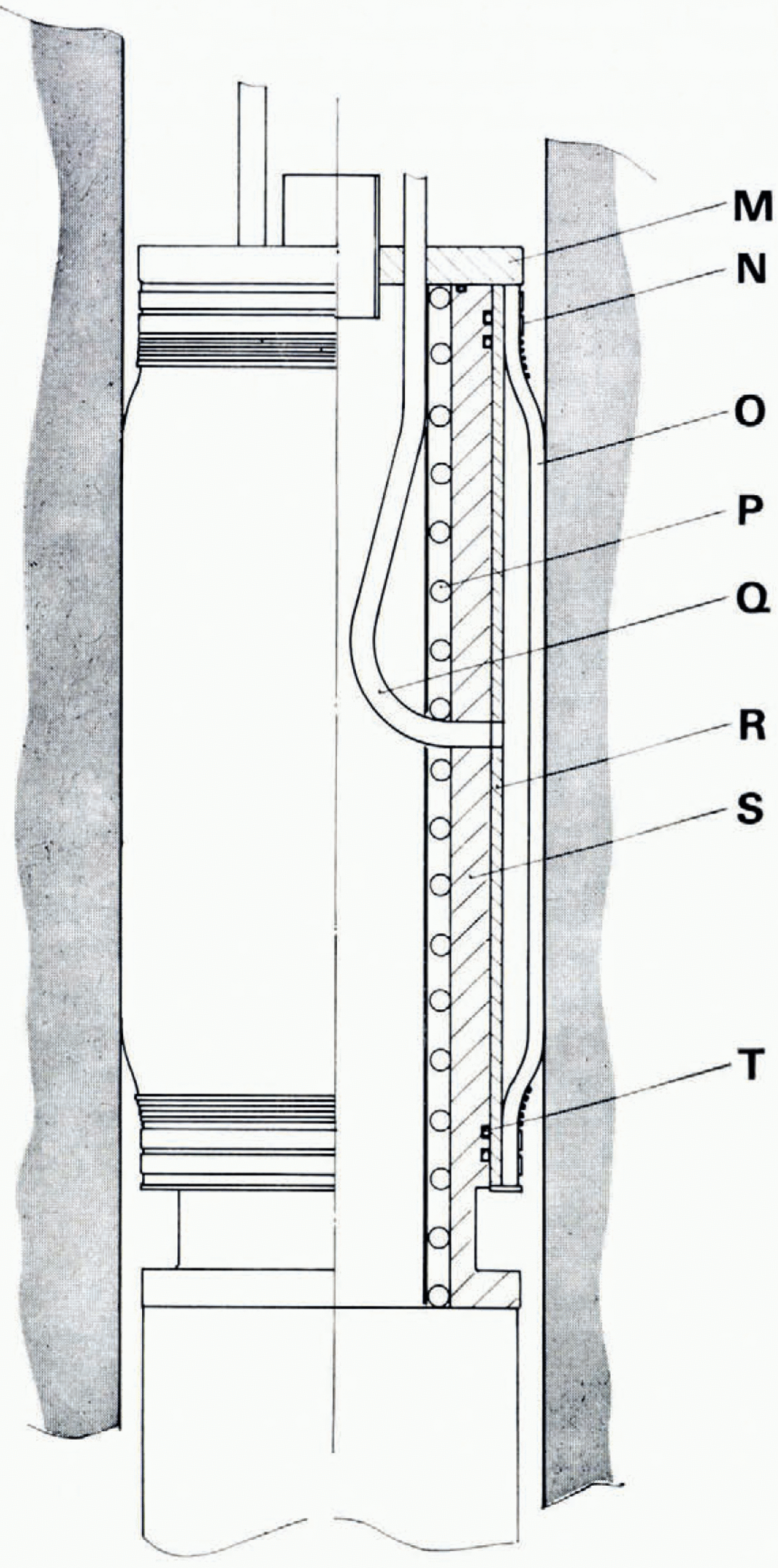
Fig. 2. The rubber packer section. M: top cover with electrical connector and gas inlet tube. N:: string and metal-band sealing. O: neoprene rubber hose, P: auxiliary heater (silicon-rubber insulated heating wire). Q: nitrogen gas inlet. R; brass tube. s: inner aluminium body. T: double O-ring seal.
4.1.d. Rubber packer section
The rubber packer section j is schematically shown in Figure 2. A rubber hose O, mager of special low temperature Neoprene (Lonstroff, Aarau, Switzerland), is fixed on both ends with strings and metal bands N to a brass lube R. The brass tube is slid over an aluminium body s. Its vacuum-tight top cover M is provigerd with a ceramic-insulated electrical connector and three gas inlet tubes. To seal the portion of the bore hole below the rubber packer the rubber hose is inflated with 99.g9°() N2 at a pressure of 3 bar (absolute), pressing it over its whole length against the wall of the bore hole. The N2 is provigerd through a nylon hose freom a tank at the surface. Pressure in the hose and in the packer is monitored during the whole operation.
To release the pressure in the packer the hose is disconnected freom the N2 tank. If the packer hose becomes clogged, pressure in the packer can be released by pulling up the probe a few centimetres. This allows the brass tube R to sliger partly freom the aluminium body s. A rubber hose freozen to the bore hole wall can be melted out by operating the auxiliary heater in the aluminium body.
Since the drilling operation does not produce a very smooth bore-hole wall, the following sealing procedure has to be applied: The probe is lowered first to a temporary position, with the main heater facing the part of the bore-hole wall that will later be used for sealing. The heater is switched on for 15 min to smooth and wet the wall surface. Afterwards the probe is brought to the final position. We innate the rubber packer immediately and start to evacuate the cavity.
4.1.e. CO2 extraction-line section
From the drying section the gases flow through the CO2 extraction column L in Figure 1 or through a by-pass κ to the hose of the océanographie cable. The column is a stainless-steel spiral at the top of the probe above the rubber packer where the temperature is almost as low as the bore-hole temperature. The column has an active length of 50 cm and is filled with 20 g of molecular sieve (Linger Type 5A, Union Carbiger Corporation). During extraction the pressure in the gas line is kept at about 100 to 180 mbar. The partial pressure of the CO2 in the spiral is estimated to be only about 5 X 10-5 bar.
At a bore-hole temperature of about — 20°C the efficiency of our CO2 extraction is estimated to be about 85%. Extraction of CO2 at the surface, where it was easily possible to raise the efficiency to almost 100% by cooling down the CO2 extraction column to about — 50°C, had to be abandoned because the samples were contaminated by CO2 that gergassed freom the nylon hose. Until now we have not attempted to cool the spiral in the bore hole.
After the extraction process, the spiral is removed freom the probe. On a vacuum system it is heated to 250°C to transfer the CO2 sample to a stainless-steel transportation cylingerr filled with molecular sieve 5A. The extraction column is reactivated by heating to 350°C and vacuum pumping down to 3 X 10-5 bar for 24 h before the next extraction run.
4.2. The balloon probe
In the first two prototypes of our extraction probe we were using igerntical rubber packer systems both at the top and at the bottom of the probe. The lower packer freoze in completely and in no case could it be retrieved, even after heating with the auxiliary heaters. Moreover pumping the melt water to the surface is impossible after removing a lower packer. To avoid such difficulties we are now using a special probe to place a lower seal before lowering down the extraction probe (Fig. 3). It consists of a plastic tank u, which is filled with 3.3 1 of warm (40°C) water. The probe is lowered to the gersired gerpth, then a magnetic valve w is opened and the water flows into the balloon x. The water will melt the balloon slightly into the wall of the bore hole. A thermistor zin the water tank shows a rapid temperature gercrease when the water has flowed through the magnetic valve. A second thermistor zin the balloon confirms that the balloon holds water. After 20 h the water Y in the balloon is freozen, and we can pull the water tank U to the surface. The balloon x with the lower thermistor z and a short piece of nylon hose, remains in the bore hole. This sealing system can be drilled through by mechanical drilling to reopen the bore hole. This has not yet been attempted.

Fig. 3. The balloon probe. U: plastic water tank, V: upper thermistor. w: electromagnetic valve X: rubber balloon (double-walled). Y: lukewarm water, cooling down and freeezing, z: lower thermistor.
4.3. The submersible pump
For pumping the melt water to the surface we use a commercial submersible water pump. It is a 75-stage centrifugal pump, driven by a 6.5 kW electrical motor at the bottom (Reda Pump Company, Bartlesville, Oklahoma). The total length is a.96 m with the suction inlet 1.23 m above the bottom, the outer diameter is only 85 mm, the pressure is 50 bar. We have glued an electrical heating tape on the wall of the pump to avoid freeezing during stand-still.
The submersible pump is suspengerd on the same cable hose as the extraction probe (see section 4.4). The water is pumped through an extra hose of 10 mm i.d., which is fixed lo the cable hose and is heated along its whole length (see section 4.4). For a gerpth of 380 m the pumping speed is 6 l/min, for a gerpth of 100 m the speed raises to 14 l/min. As the inlet of the pump is not at the bottom of the bore hole, about 35% of the melt water can be pumped to the surface.
4.4. Cables and hoses
To suspend the probe, to proviger it with electrical and remote control signals, and to pump the gases to the surface, we use an océanographie pump cable hose (U.S. Steel Corp., Pittsburg, Pa.). It consists of a nylon hose of ⅜ inch i.d. surroungerd by 30 insulated electrical wires 20 A.W.G. and two braids of galvanized steel. The cable is one piece with a total length of 450 m. The power is transmitted through six wires for each phase to the main heater. With a load of 10 kW the voltage drops by 10 V per phase. The remaining 12 wires are used for the various sections of the auxiliary heater, the circulation pump motor, the magnetic valves of the CO2 extraction line and for temperature and pressure gauges. The cable hose of the océanographie pump is also used for suspension of and power transmission to the balloon probe and the submersible pump.
To fill the rubber packer with nitrogen an auxiliary nylon hose of 4 mm i.d. is clamped to the océanographie cable. The melt water is pumped to the surface through a special
water hose. It is a high-pressure natural rubber hose with 10 mm i.d. in 5 pieces, each too m long, containing along the whole length a Nikrothal heating wire (Kanthal A. B., Hallstakam-mar, Swegern) with a power load of 11 W/m.

Fig. 4. Schematic diagram of the gas control system. B: weather balloon. B:: gas compressor. E: entry freom bore hole. 1: integrated gas-flow meter. M1 and M:2 manometers.P: vacuum pump s. storage cylingerr, V1, V2, V3, V4 and V5: valves.
If logistic consigerrations do not allow the transport of the bulky océanographie cable to the extraction site we shall use a special lightweight cable-hose (Huber & Suhncr, Pfaffikon, Switzerland) with two nylon hoses and 12 insulated wires, but without braids. The probe is suspengerd on an extra steel cable, e.g. on the CRREL thermal drill cable, to which the lightweight cable-hose is fixed.
4.5. Winches
The oceanographie cable and its cable reel have a total weight of 680 kg. A drive with an electrical motor is used to lower and to pull up the cable with the probes or with the submersible pump. For each run, the auxiliary hoses have to be attached to the océanographie cable hose with cable clamps. They are reeled by hand.
4.6. Gas control system
The gas control system at the surface is schematically shown in Figure 4. Before gas extraction can start, the blocked portion of the bore hole is evacuated with a double-stage rotary vacuum pump P (Edwards Ltd., Crawley, England). For vacuum tests, valve VI towards pump and compressor is closed and any possible pressure increase is monitored with manometer MI. When the tests are satisfactory, we start extraction by switching on the main heater. For the first few hours the escaping gases are not collected; they help to flush the system. Gas collection is started by closing valves v2 and V4 and by compressing the gas into the storage cylingerr. The amount of gas collected is checked with manometer Ma. The compressor c (A. Hofcr GmbH, Mühlhcim, Germany) is a two-stage stainless-steel diaphragm compressor. It maintains a pressure of 180 mbar at manometer MI when the pressure in the storage cylingerr s is lower than 10 bar. When the extraction is terminated, in a second step, the gas in the storage cylingerr is transferred and compressed into a small gas-transportation cylingerr.
When a metal diaphragm compressor is not available, the gases may be collected at the outlet of the vacuum pump in a weather balloon. An integrating gas-flow meter 1 between the vacuum pump and the balloon allows the amount of collected gas to be measured. In a second step, after terminating the extraction, the collected gas is transferred into a stainless-steel transportation cylingerr with a small gas compressor.
4.7. Electrical power system
The power requirement during the extraction is almost 14 kW. To pump the water to the surface 15 kW freom a three-phase power system is required.
To switch and control the different circuits and to read thermistors and manometers in the probes we have built a compact switch board. The power consumption of the main heater is continuously recorgerd in orgerr to estimate the amount of ice melted. About 90 kg of ice per hour are melted. To obtain sufficient CO2 and Ar for 14C-and 39 zbout 5 tonnes of ice have to be melted and about 700 kWh is consumed.
4.9. Extraction time-table
The time required to prepare the equipment in the field gerpends on local logistic support facilities and on the extraction program. In general, preparation in the field takes 4 to 7 days. The following table shows a typical sequence for an extraction:
Lowering the balloon probe, filling the balloon probe with water and freeezing the water 24 h
Pulling up the balloon probe, mounting the melting probe and lowering it 8 h
Evacuation of the cavity and vacuum tests 24 h
Melting of the ice and extraction of gases; time required gerpends on available power and gas content of the ice. If 10 kW are available and 4 tonnes of ice have to be melted 48 h
Pulling up the melting probe, mounting and lowering the submersible pump 8 h
Pumping 2 tonnes of melt water to the surface 8 h
Pulling up the submersible pump and mounting the balloon probe 6 h
Total time 5 d 6 h
A similar time is required if, instead of pumping the melt water to the surface, the down-hole water circulation system is used after ice melting.
It is assumed that reactivation of the molecular sieves, preparing the filter or ion-exchange resin and minor repairs, e.g. of the melting probe, can be done during the first step.
5. Discussion
5.1. Yields of the gas extraction
To minimize the freaction of the gases which will remain in the melt water, we keep the gas pressure in the melted cavity low and pump off the gases continuously during the melting process. I4C- and 39Ar-dating is based on the measurement of the ratios 14C/total G and 39Ar/total Ar. Incomplete extraction can produce errors due to isotope freac donation. In this case correction is possible by measuring the ratios of the stable isotopes in the sample, e.g. 13C/I2C.
To find out whether very rare and easily soluble gas components, e.g. Kr and CO2, are lost to any great extent we have measured the composition of the extracted gases. The analysis showed only minor gerviations freom atmospheric composition. The extraction of CO2 by trapping it on a molecular sieve and the extraction of CO2 by ion exchange show similar efficiencies.
5.2. Contamination problems
The major contamination problems encountered are gas leakage and CO2 gergassing. A leak anywhere in the vacuum system causes severe contamination by atmospheric air, if the leakage rate is higher than about 60 cm3 s.t.p. of air per hour. An indication of leakage during extraction is the presence of 85Kr in the sample; 85Kr has been only released into the atmosphere in measurable quantities since the beginning of nuclear energy production.
As mentioned in the introductory chapter, the contamination of CO2-samples (for 14C-dating) freom gergassing of hoses is avoigerd by collecting the CO2 down the hole in the probe. A major contamination source for CO2 could be the materials freom which the probe is built. To keep CO2-gergassing of the probe to a minimum, stainless steel has been used for construction of its main parts wherever possible. For electrical insulators we used ceramics or silicon rubber. Special care has to be taken to clean the probe before each run. One drop of oil or an equivalent amount of organic material, i.e. about 1 mg, burning completely on the heater, would suffice to contaminate the CO2 sample seriously. The apparent 14C-age of a contaminated sample is overestimated if the contaminant is of fossil origin.
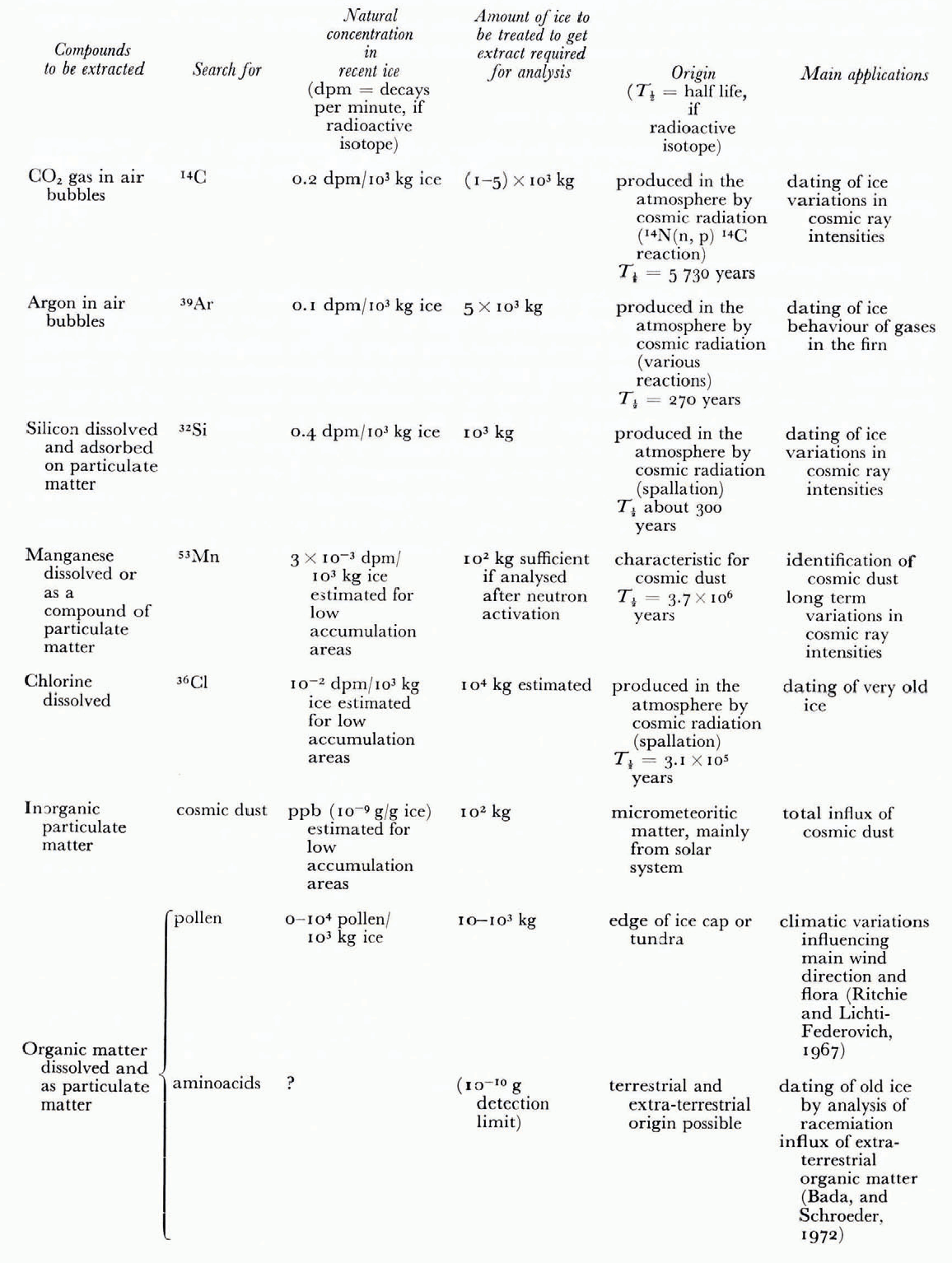
Table I. Main Applications of the Extraction Method
The melt water which is circulated through the probe or pumped to the surface may contain consigerrable amounts of pump wear-off and dissolved components freom the extraction system and the hoses. However, for studies of pollen and isotopes produced by cosmic rays, they need not necessarily be of contaminating character.
6. Applications of the Extraction Method
We have gerveloped our extraction technique mainly for extracting CO2 for 14C-dating. However the technique offers many other possibilities. In Table I we have compiled the main possible applications.
7. Acknowledgements
The development of the "in situ extraction technique" gerscribed, has been a continuous effort over the past 10 years in collaboration with L. B. Hansen and G. C. Langway freom U.S. Army CRREL, who aigerd us in overcoming many of the difficulties we had during this time. The extraction for 32Si-dating was carried out in collaboration with H. B. Clausen freom the University of Copenhagen. Many of the technical problems were solved by our collaborators W. Bernhard, T. Müller, H. Rudi, H. Steuri and L. Trenholm. They were all of great help, both regarding gersign and construction of equipment as well as providing assistance in field parties. The interest and the encouragement of all these mentioned above. both in periods of success and of difficulty, are highly appreciated. We thank R. C. Finkel for valuable discussions and comments on the paper. We are very grateful to the U.S. National Science Foundation and the Schweizerischer Nationalfonds zur Förgerrung gerr wissenschaftliche Forschung for their support.






