Introduction
Sheep haemonchosis is considered to be one of the main health problems affecting sheep flocks worldwide (Fiaz-Qamar et al., Reference Fiaz-Qamar, Maqbool and Ahmad2011). Infected animals show clinical signs such as anorexia, weight loss, diarrhoea, submaxillary oedema, slow growth, malnutrition (Prakash & Bano, Reference Prakash and Bano2010) and even death of young animals (Garedaghi et al., Reference Garedaghi, Hashemzadefarhang and Esmaeli2013). Haemonchosis and other gastrointestinal parasitic nematodes (GIPN) have been controlled mainly with chemical anthelmintic drugs that are administered as a continuous treatment strategy for de-worming animals. However, this system has led to the development of anthelmintic resistance (AR) (Torres-Acosta et al., Reference Torres-Acosta, Mendoza-de-Gives, Aguilar-Caballero and Cuéllar-Ordaz2012) as well as health risks due to the possible persistence of chemical residues in animal products. The use of ethnoveterinary or traditional medicines in veterinary practice has gained the attention of numerous researchers worldwide, and some plants have been reported to be excellent candidates for controlling animal parasitosis caused by nematodes (Akerreta et al., Reference Akerreta, Calvo and Cavero2010; Bharati & Sharma, Reference Bharati and Sharma2012; Hassan et al., Reference Hassan, Murad, Tariq and Ahmad2014; Saha et al., Reference Saha, De Sarker and Sen2014). Plants such as Trifolium repens and Vernona anthelma are currently being investigated as possible sources of agents to control GIPN in ruminants (Heckendorn et al., Reference Heckendorn, Häring, Maurer, Senn and Hertzberg2007; Alawa et al., Reference Alawa, Adamu, Gefu, Ajanusi, Abdu and Chiezey2010). Oxalis tetraphylla is a bulbous plant from Mexico and Guatemala that belongs to the Oxalidaceae family, which has approximately 900 species (Burger, Reference Burger1991). It is widely distributed in South America, Mexico and Africa (Loudon, Reference Loudon2009). It is traditionally called ‘Trebol de la Buena Suerte’ (good luck clover) and possesses four triangular green leaves with purple colour diffused at the centre of the leaves (Brickell & Zuk, Reference Brickell and Zuk1997) (fig. 1). Although this plant has been used for human consumption, it is mostly used for cattle grazing (Romero, Reference Romero2000). The most reliable assay for assessing the in vivo anthelmintic activity of plant extracts involves slaughtering the animal and then counting the adult worms recovered from the digestive tract. However, this method requires the euthanization of numerous animals, which is very expensive. Another technique that allows the identification of potential anthelmintic compounds is based on counting the number of eggs/gram of faeces (epg) before and after treatments (Irum et al., Reference Irum, Ahmed, Mukhtar, Mushtaq, Mirza, Donskow-Łysoniewska, Qayyum and Simsek2015). The method is simple, inexpensive and avoids the euthanization of animals. The present study was designed to assess both the in vitro nematocidal activity against Haemonchus contortus infective larvae (L3) and the in vivo effect of oral administration of O. tetraphylla hydroalcoholic extract (HE) on the faecal egg count in lambs experimentally infected with H. contortus.

Fig. 1. Leaves of the leguminous plant Oxalis tetraphylla collected from the top of the Woodland Mountain at San Juan Tlacotenco village, Tepoztlán, Morelos State, Mexico.
Materials and methods
Plant material
Wild specimens of O. tetraphylla were collected from a forest at the top of the ‘Cerro light’ mountain (mountain of light) in the village of San Juan Tlacotenco, Tepoztlán Municipality, Morelos, Mexico, in January 2012. A voucher specimen of the plant material was deposited at the herbarium of the National Institute of Anthropology and History of Mexico, Cuernavaca City, Morelos State, Mexico (registration number: 2058). A biologist, Margarita Aviléz, established the taxonomy of the plant specimen.
Extract preparation
Five kilograms of the wild leaves and stems were dried and then extracted three times by maceration in an ethanol–water mixture (60:40, v/v, 15 litres; Merck, Darmstadt, Germany) for 24 h at room temperature (18–25°C). This maceration process was described previously by De Jesús Gabino et al. (Reference De Jesús-Gabino, Mendoza de Gives, Salinas-Sánchez, López-Arellano, Liébano-Hernández, Hernández-Velázquez and Valladares-Cisneros2010). The HE of each plant material sample was concentrated and completely dried using a rotatory evaporator (Heidolph Laborota 4000; Heidolph Instruments, Schwabach, Germany) under reduced pressure at 50–60°C to obtain 38.1 g of HE extract.
Phytochemical analysis using thin layer chromatography
To identify the chemical compounds in the O. tetraphylla HE, we used a thin layer chromatography (TLC) technique to analyse specific chemical reactions (Wagner et al., Reference Wagner, Bladt and Zgainski1984). We used a visualization agent (2-aminoethyl diphenylborinate in polyethylene glycol) to detect flavonoids, which were observed under ultraviolet (UV) light at a long wavelength (360 nm). A comparison of the TLC analysis of samples previously isolated indicated that the detected compounds were β-sitosterol and stigmasterol sterols, which are known to have widespread occurrence in the plant kingdom. The compounds were revealed using the Komarowski reagent (4-hydroxybenzaldehyde in sulphuric acid).
Phytochemical analysis using high-performance liquid chromatography
The high-performance liquid chromatography (HPLC) analysis was performed using a Waters 2695 separation module system equipped with a Waters 2996 photodiode array detector and the Empower Chromatographic Manager version 1 software (Waters, Milford, Connecticut, USA). The analysis was performed using a Merck Superspher® RP-18 (Merck) column (5 μm, 100 mm). The mobile phase consisted of a gradient system of water, trifluoroacetic acid (TFA 0.5%, solvent A) and acetonitrile (solvent B). The chromatographic process was run for 28 min on the following schedule: 1–2 min (solvent A:B, 100:0), 3–4 min (90:10), 5–7 min (80:20), 8–14 min (70:30), 15–18 min (60:40), 19–22 min (20:80), 23–26 min (0:100) and 27–28 min (100:0). The flow rate was 1 ml/min while the sample was injected in a volume of 10 μl and at a 1 ml/min flow rate. The detection wavelength was 190–400 nm.
Pharmacological evaluation
Two experiments were performed. Experiment 1 was aimed at evaluating the in vitro larvicidal activity of the O. tetraphylla HE against the L3. The second experiment evaluated the effect of oral administration of O. tetraphylla HE to lambs on the reduction of numbers of H. contortus eggs eliminated in the faeces.
Biological material
L3 processing
Faecal samples of sheep experimentally infected with H. contortus were processed by preparing faecal cultures and extracting the infective larvae using a Baermann's funnel. The L3 were washed several times using density gradients of a 40% saccharose solution, rinsed and then suspended in sterile water.
Experiment 1: in vitro assessment of effects of O. tetraphylla HE against L3
Experimental design
Two experimental stages were used to assess the in vitro activity of the O. tetraphylla HE against sheathed and exsheathed L3. The larvae/extract treatment was carried out in 96-well plates. Three wells per treatment were considered as three experimental units. The design of each experimental stage was similarly structured, and three series of three wells were treated as follows. Stage 1: first, 20 μl of water containing 200 sheathed L3 were placed in every well of the three series. Series 1 contained only larvae; in series 2 and 3, 80 μl of 1% ivermectin or O. tetraphylla HE at a concentration of 20 mg/ml were added and mixed. Stage 2 followed the same procedure as that used in Stage 1 except that exsheathed larvae were used.
Two incubation periods were used (24 and 48 h post-treatment). After incubation, ten 5-μl aliquots (considered as n = 10 aliquots/well from three experimental units per treatment) were placed on a slide and observed under a microscope at 4× and 10× magnifications. The total, dead and live larvae were counted. The criteria for identifying the dead and live larvae were based on their mobility/immobility; physical stimuli were applied to confirm the dead and live larvae. The proportions of dead and live larvae were estimated for each series. The proportion of live larvae in series 1 (control, water) was considered as 100% and used for comparison. Ponder adjustments were performed when necessary considering the number of larvae that died for reasons other than the treatments.
Statistical analysis
The data were transformed (√x + 0.5) using a completely random design. The means of the live larvae were compared using an analysis of variance (ANOVA), and the complementary Tukey's test was performed to identify the differences between treatments. The statistical analysis software (SAS) program (version 8) was used (SAS Institute Inc., Cary, North Carolina, USA). The percentage in vitro efficacy of the extract was estimated using the following formula (Eguale & Giday, Reference Eguale and Giday2009):
 $$\% {\rm Efficacy} = \displaystyle{{\matrix{{\bar {\rm x}} \; {\hbox {live larvae in control group}} \cr - {\bar {\rm x}}\; {\hbox {live larvae in treated group}}}} \over {{\bar {\rm x}} \; {\hbox {live larvae in control group}}}} \times 100$$
$$\% {\rm Efficacy} = \displaystyle{{\matrix{{\bar {\rm x}} \; {\hbox {live larvae in control group}} \cr - {\bar {\rm x}}\; {\hbox {live larvae in treated group}}}} \over {{\bar {\rm x}} \; {\hbox {live larvae in control group}}}} \times 100$$Experiment 2: evaluation of the effect of oral administration of O. tetraphylla HE on the number of H. contortus eggs eliminated in sheep faeces
Experimental groups of animals
Twenty-seven Pelibuey lambs aged 4–6 months, previously infected with 350 L3/kg body weight (BW), were randomly assigned to three groups of nine lambs each. Group 1 was treated with water (positive control); Group 2, levamisole, 7.5 mg/kg BW (one single dose); and Group 3, O. tetraphylla HE, 20 mg/kg BW (orally administered daily for 8 days). The animals were maintained in individual paddocks and received a dried alfalfa nutritional regime and water ad libitum. The experimental groups were established based on their epg counts on day −3 and treatments were administered on day 0.
Faecal sampling
Faecal samples were collected directly from the rectum of each lamb on days −3, 0, 2, 4, 7, 9 and 11 of the experiment. The average number of nematode eggs eliminated (epg) by each lamb in every group was estimated using the McMaster technique (Paul et al., Reference Paul, Torgerson, Höglund and Furrer2014).
Statistical analysis
The data were analysed using the SAS program. The epg values were 10log transformed (epg + 1) to achieve a normal distribution approximation (Bouix et al., Reference Bouix, Krupinski, Rzepecki and Nowosad1998) and an analysis of repeated measures over time series was performed. A Duncan's multiple range test was used to compare the means, and the following statistical model was used:
where Y ijkl = variable/response (epg), μ = general mean, E i = ith effect/sample day (i = 11), T j = jth effect/treatment (j = 4), (E × T)ij = interaction effect between treatments/sampling days, A k = random effect of the kth lambs, εijk = experimental error.
Results
Experiment 1
In the in vitro assay, the proportions of dead and total larvae (sheathed and exsheathed) recovered after incubation for 24 and 48 h with the different treatments are shown in tables 1 and 2, respectively. After a 24-h incubation, the mortality rate of the sheathed larvae in series 1 (control, water) was low and no mortality was observed for exsheathed larvae. In series 2 (control, ivermectin), no live sheathed or exsheathed larvae were observed. However, when the mortality of sheathed larvae was compared with that of its control (water-treated), a 97.6% ponderate mortality (real mortality) was recorded. In series 3, (O. tetraphylla HE), 80.9% and 97% ponderate mortalities were observed for the sheathed and exsheathed larvae, respectively (table 1). After a 48-h incubation, a low mortality was observed in the sheathed larvae of the control (series 1). No mortality was observed in the exsheathed larvae of the control series. Series 2 (control, ivermectin) exhibited a 100% mortality in both sheathed and exsheathed larvae, while series 3 (O. tetraphylla HE) showed corresponding ponderate mortalities of 86.87 and 98.85%, respectively (table 2).
Table 1. Proportion of dead and total Haemonchus contortus infective larvae (L3, sheathed and exsheathed) exposed to Oxalis tetraphylla hydroalcoholic extract (HE) after 24-h incubation, and mortality percentages.
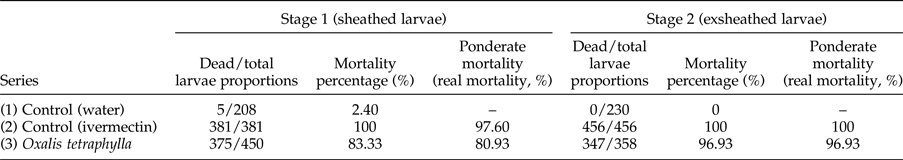
n = 3 (3 wells, 10 aliquots per well). P < 0.05.
Table 2. Proportion of dead and total Haemonchus contortus infective larvae (L3, sheathed and exsheathed) exposed to Oxalis tetraphylla hydroalcoholic extract (HE) after 48-h incubation, and mortality percentages.
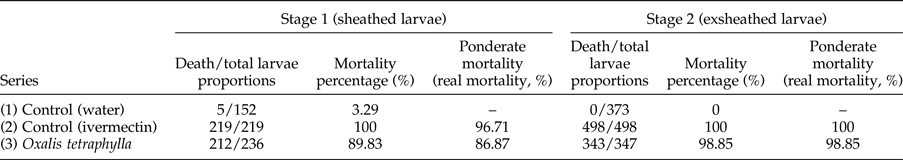
n = 3 (3 wells, 10 aliquots per well). P < 0.05.
Experiment 2
The results of experiment 2, including the mean number of H. contortus eggs eliminated per group of animals and the egg reduction percentage in the different groups relative to the control group are shown in fig. 2. The overall results of group 1 (control, water) showed the highest epg values. In group 2 (levamisole), no eggs were recorded in the faecal samples, indicating a 100% efficacy, while group 3 (O. tetraphylla HE) showed variable reduction percentages ranging between 23.1 and 63.7%. The maximum values were obtained on day 11 after treatment. The overall reduction percentage at the end of the experiment for the O. tetraphylla HE was 45.6% (fig. 2, P < 0.05). Statistical differences were found between the control group treated with water (positive control) without any de-worming treatment and the extract-treated group (P < 0.05). However, this group exhibited a lower effect than that of the levamisole-treated group (fig. 2).
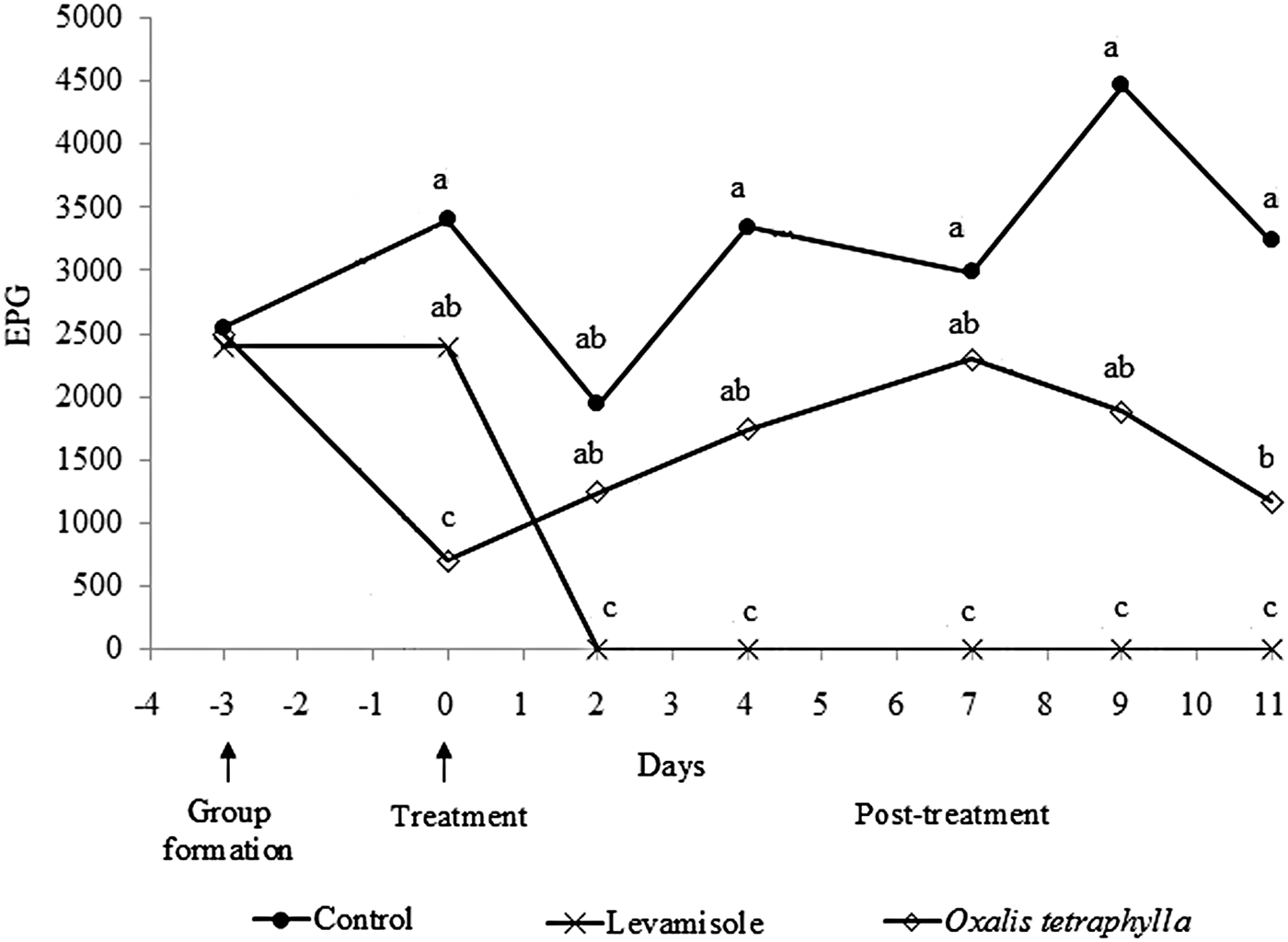
Fig. 2. Haemonchus contortus egg counts per gram faeces from lambs receiving the three different treatments.
Analysis of phytochemicals
The phytochemical analysis of the O. tetraphylla HE showed a positive reaction to the reagent that revealed the presence of classic flavonoids and sterols. Figure 3 shows the HPLC chromatogram generated at 345 nm, indicating a mixture of compounds corresponding to flavonols according to UV absorption (218, 272 and 357 nm).
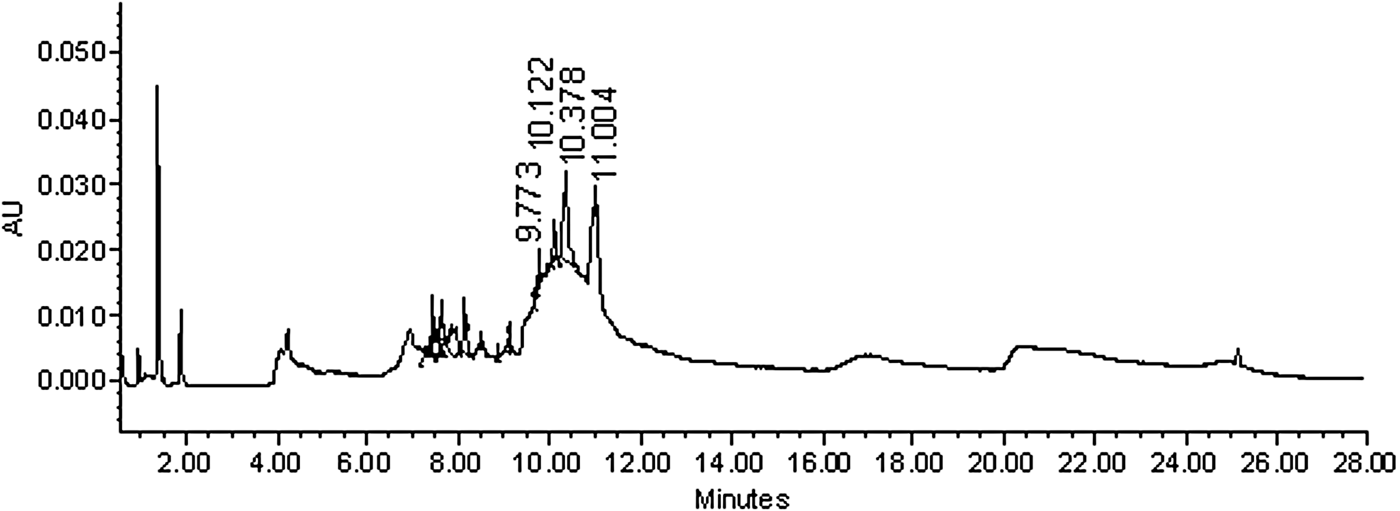
Fig. 3. Chromatogram generated from an Oxalis tetraphylla hydroalcoholic extract showing several compounds identified at different times. Compounds were eluted between 2 and 12 min. Compounds reaching values higher than 350 nm corresponded to flavonoids and sterols.
Discussion
Plants contain numerous beneficial nutritional compounds and are used as an important source of protein, starch, minerals and vitamins. Furthermore, they are also considered to contain medicinal compounds. including phenols, inositol phosphates and oligosaccharides, that are beneficial to cattle and small ruminants (Schuster-Gajzágó, Reference Schuster-Gajzágó and Fuleky2004). To date, very little information is available about the medicinal effects of the plant, O. tetraphylla (Oxalidaceae). The results of the present study showed the potent in vitro nematocidal activity of O. tetraphylla HE against both sheathed and exsheathed L3 after 24- and 48-h incubations. These results encouraged us to further investigate the possible anthelmintic effect of the O. tetraphylla HE in sheep infected with H. contortus.
This study provided evidence of the presence of bioactive compounds with anthelmintic activity in O. tetraphylla. Specifically, oral administration of the HE to H. contortus-parasitized sheep reduced the epg values to approximately 50% after daily treatment for 8 days. This level of reduction is considered inadequate by the World Association for the Advancement of Veterinary Parasitology (WAAVP) (Coles et al., Reference Coles, Jackson, Pomroy, Prichard, Himmelstjerna, Silvestre, Taylor and Vercruysse2006). Nevertheless, it is important to consider that this low epg reduction was obtained with a crude extract, and further purification could lead to the identification of a more potent molecule with improved effects. Our extensive search for relevant information did not reveal any reports of the possible ovicidal effects of O. tetraphylla extracts against any nematode. Therefore, this could be the first report on this activity in O. tetraphylla.
Furthermore, the hypothesis that this plant extract has a lethal effect against adult parasites in sheep following oral administration into the abomasum was proven. The common assay to determine the precise antiparasitic effect of plant extracts or chemical compounds involves collecting, counting and comparing the number of adult parasites in untreated (control) and treated sheep at necropsy. However, this method is expensive and requires the euthanization of the animals, as previously mentioned. Therefore, we assessed the treatment effects by determining and comparing the epg faecal reduction in treated and control groups (untreated sheep).
The low reduction in the epg values could be attributable to the use of a crude plant extract that contained numerous bioactive compounds that could interfere with the biological activity of the extract against parasites. The chromatographic purification of the extract could possibly lead to the identification of molecules with higher efficiency against the parasites than that of the crude extract. The epg reduction percentage of the O. tetraphylla HE (< 50%) was not as expected; however, we believe that purifying the plant extract could lead to the isolation of a component with a higher epg reduction percentage. Furthermore, this compound could be a useful tool in an integrated control programme including other alternative options, focused on reducing the parasitic burden through different mechanisms. For example, strategies such as the nutritional strategy of improving the quality and quantity of protein and metabolizable energy levels consumed (Yap et al., Reference Yap, Utzinger, Hattendorf and Steinman2014), and management measures such as rotational grazing (Burggraaf et al., Reference Burggraaf, Boom, Sheath and Brooky2009; Benson, Reference Benson2012) or alternated grazing with different host species (Marshall et al., Reference Marshall, Gebrelul, Gray and Ghebreiyessus2012), are beneficial. Furthermore, the use of natural nematode enemies such as nematophagous fungi of the species Duddingtonia flagrans, vaccines (Roberts et al., Reference Roberts, Antonopoulos, Haslam, Dicker, McNeilly, Johnson, Dell, Knox and Britton2013; Fawzi et al., Reference Fawzi, González-Sánchez, Corral, Cuquerella and Alunda2014), genetic selection of animals resistant to parasites (Hutchings et al., Reference Hutchings, Knowler, McAnulty and McEwan2007; Periasamy et al., Reference Periasamy, Pichler, Poli, Cristel, Cetra, Medus, Basar, Thiruvenkadan, Ramasamy, Ellahi, Mohamed, Teneva, Shamsuddin, García and Diallo2014), and copper particles (Sayward & Sayward, Reference Sayward and Sayward2014) are other promising measures. In addition, other groups of plants have also been explored under different conditions to identify additional natural alternatives for control, and different results have been published (table 3).
Table 3. In vitro and in vivo effect of different plants against sheep gastrointestinal parasitic nematodes.

In conclusion, the present study provides relevant information about the presence of anthelmintic compounds in the crude HE of the bulbous plant, O. tetraphylla. Furthermore, the type of flavonoids identified killed the infective stages of H. contortus in in vitro assays and reduced the H. contortus egg nematode population in the faeces of treated animals. The results of this study could be used as a reference for further investigations focused on identifying a biomolecule with anthelmintic activity from the bulbous plant, O. tetraphylla, for the control of sheep haemonchosis.
Acknowledgements
This research formed part of the theses of B.J.G.-C. and M.R.-L., submitted towards the qualification of Biotechnologist Engineers Labastida, under the direction of P.M. de G. at the Universidad Politécnica del Estado de Morelos, Jiutepec, Morelos, Mexico.
Financial support
The present research received financial support from Instituto Nacional de Investigaciones Forestales, Agricolas y Pecuarias (INIFAP)-México (Fondos Fiscales-2012) from the Project ‘Assessment of four plant extracts as phytotherapeutics against sheep parasitic nematodes’.
Conflict of interest
None.
Ethical standards
The sheep were strictly maintained under the Norma Oficial Mexicana (Official Rule Number) NOM-051-ZOO-1995 (http://www.senasica.gob.mx) and the LEY Federal de Sanidad Animal (Federal law for animal health) DOF 07-06-2012 (http://diputados.gob.mx/LeyesBibliop/ref/lfsa.htm). These guidelines specify that all the procedures performed in studies involving animals must follow the Federal Law and Official Rule strictly in accordance with the ethical standards of INIFAP. Furthermore, the guidelines are based, in part, on the Guide for the care and use of laboratory animals published by the Institute of Laboratory Animals Resources Commission on Life Sciences, National Research Council, 1996.








