Introduction
Human cystic echinococcosis (CE) is a widespread, chronic, endemic, helminthic zoonosis caused by larval forms (metacestodes) of tapeworms of the genus Echinococcus and it is a major global health problem to date (Yang et al., Reference Yang, Ellis and McManus2012). CE is characterized by cystic lesions, most commonly in the liver and lungs, which can be fatal if not treated with surgery and/or chemotherapy (Eckert & Deplazes, Reference Eckert and Deplazes2004; Budke et al., Reference Budke, Carabin, Ndimubanzi, Nguyen, Rainwater, Dickey, Bhattarai, Zeziulin and Qian2013). The growth of hydatid cysts may lead to oppressive symptoms such as abdominal distension and stomach ache and, at the final stage, these symptoms may become severe and even fatal. The incidence rate of echinococcosis in China is the highest in the world. The disease remains endemic in many areas, and Xinjiang, Qinghai, Gansu and Ningxia are the most severely affected regions. A national survey revealed that the serological prevalence and morbidity in 12 provinces and autonomous regions were 12.0% and 1.1%, respectively (Yu et al., Reference Yu, Wang, Wu, Ma, Liu, Liu, Zhao, Morishima and Kawanaka2008).
The World Health Organization Informal Working Group on Echinococcosis (WHO-IWGE) proposes four different treatments for CE: (1) surgery; (2) puncture, aspiration, injection of protoscolicidal agent and re-aspiration; (3) chemotherapy with the benzimidazole compounds albendazole (ABZ) or mebendazole (MBZ); and (4) watch and wait for inactive, clinically silent cysts. Although surgical treatment is the primary option, all patients need drug treatment before or after surgery. For some patients for whom surgery is contraindicated, chemotherapy is the only modality of treatment (McManus et al., Reference McManus, Gray, Zhang and Yang2012). The main clinical drugs for echinococcosis are ABZ and MBZ. However, these drugs can cause severe adverse reactions, including systemic nervous, digestive and gastrointestinal diseases (Rajshekhar, Reference Rajshekhar1998; Shirokova & Chebyshev, Reference Shirokova and Chebyshev2003; Njomo et al., Reference Njomo, Tomono, Muhoho, Mitsui and Josyline2014). To date, none of the therapeutic modalities can completely cure echinococcosis. Therefore, the identification of novel and efficient treatment options is essential for the treatment of CE.
Many scholars have shown interest in traditional Chinese herb extracts as novel therapeutic options for CE. Sophora moorcroftiana is a folk-medicinal shrub endemic to the wide valleys and middle reaches of several main tributaries of the Yalu Tsangbo River in Tibet, China. In Chinese folk medicine the decoction of the seeds is used as an anti-inflammatory, detoxicant, anti-emetic and antibacterial medicine (Ma et al., Reference Ma, Li, Yin and Wang2004b). Our previous studies indicated that crude alkaloids of S. moorcroftiana seeds presented bacteriostatic (Ma et al., Reference Ma, Li, Yin and Wang2004b), antitumour (Ma et al., Reference Ma, Li, Yin, Meng, Zhou and Lu2003, Reference Ma, Li, Yin and Wang2004a) and protoscolicidal activity (Ma et al., Reference Ma, Bao, Wan, Liao, Yin, Meng, Zhou, Lu and Li2007), and had weak insecticidal activity against Pieris rapae (Ma et al., Reference Ma, Li, Wang and Yin2005). If is of interest that the combination of crude alkaloids of S. moorcroftiana seeds and ABZ has a significant additive effect for treatment of experimental echinococcosis in mice (Ma et al., Reference Ma, Bao, Wan, Liao, Yin, Meng, Zhou, Lu and Li2007).
Bacillus Calmette–Guérin (BCG) is composed of attenuated mycobacteria and is used as an effective vaccine to prevent tuberculosis. BCG is also used to treat allergic disease, tumours and immune disorders (Alexandroff et al., Reference Alexandroff, Jackson, O'Donnell and James1999; Shoenfeld et al., Reference Shoenfeld, Aron-Maor, Tanai and Ehrenfeld2001; McShane et al., Reference McShane, Pathan, Sander, Keating, Gilbert, Huygen, Fletcher and Hill2004), and has a remarkable curative effect in the treatment of bladder cancer (Alexandroff et al., Reference Alexandroff, Jackson, O'Donnell and James1999; Li et al., Reference Li, Han, Zhu, Cui, Ma and Dong2016). BCG is used as an adjuvant to modify the immune response by activating antigen-presenting cells such as macrophages and dendritic cells. Therefore, BCG contains many immune stimuli to improve the Th1-type cellular immune responses (Scanga & Le Gros, Reference Scanga and Le Gros2000) against tumours and parasites.
However, few studies have evaluated combined therapy with S. moorcroftiana alkaloids and BCG against echinococcosis. In this study, we evaluated the efficacy of the combined treatment with water-soluble alkaloids and BCG in vivo.
Materials and methods
Drug preparation
The water-soluble total alkaloids of S. moorcroftiana seeds (SMSa2) were extracted in our laboratory and the analytical thin layer chromatography results indicated that SMSa2 contained matrine and sophocarpine. All culture media were purchased from Gibco-BRL (Gaithersburg, Maryland, USA) and ABZ was purchased from Sigma (St. Louis, Missouri, USA). An enzyme-linked immunosorbent assay (ELISA) kit was purchased from Dakewei Biotech Company (Shenzhen, China). SMSa2 and ABZ were dissolved in sterile water at a final concentration of 7.5 mg/ml.
Animals and parasites
Female NIH mice aged 6–8 weeks (body weight 25–30 g) were purchased from the Institute of Biological Products in Lanzhou. The protoscoleces of hydatid cysts were removed aseptically from naturally infected sheep collected from a slaughterhouse located in Xining, Qinghai Province, China. An in vitro culture of Echinococcus granulosus protoscoleces was maintained as described previously (Amri et al., Reference Amri, Aissa, Belguendouz, Mezioug and Touil-Boukoffa2007). Briefly, the harvested protoscoleces were washed three times with saline and twice with Hanks’ balanced salt solution containing 100 U/ml penicillin and 100 μg/ml streptomycin. The cultures with a survival rate exceeding 90% were used for animal infection. The final concentration was 20,000 live protoscoleces in 1 ml of phosphate-buffered saline (PBS).
Experimental infection and design
Thirty-five female NIH mice were divided into an uninfected ‘blank’ group (n = 5) and an infected group (n = 30). The mice were infected by intraperitoneal inoculation with 5000 fresh protoscoleces/animal suspended in 250 μl sterile PBS. After 20 weeks, five infected mice were selected and sacrificed at random to confirm the presence of cysts in the peritoneal cavity. The remaining infected mice were randomly divided into five groups of five mice and received the following treatments from weeks 21 to 26. (1) ABZ group: the animals were treated with a suspension of 100 mg/kg ABZ by daily intragastric administration; (2) SMSa2 group: the animals were treated with a suspension of 100 mg/kg SMSa2 by daily intragastric administration; (3) BCG group: the animals received an abdominal subcutaneous injection of 5 × 106 CFU BCG at weeks 21 and 24; (4) SMSa2 + BCG group: the animals were treated with SMSa2 and BCG as described above; and (5) untreated group and control uninfected group: the animals received saline by intragastric administration as a placebo (fig. 1).
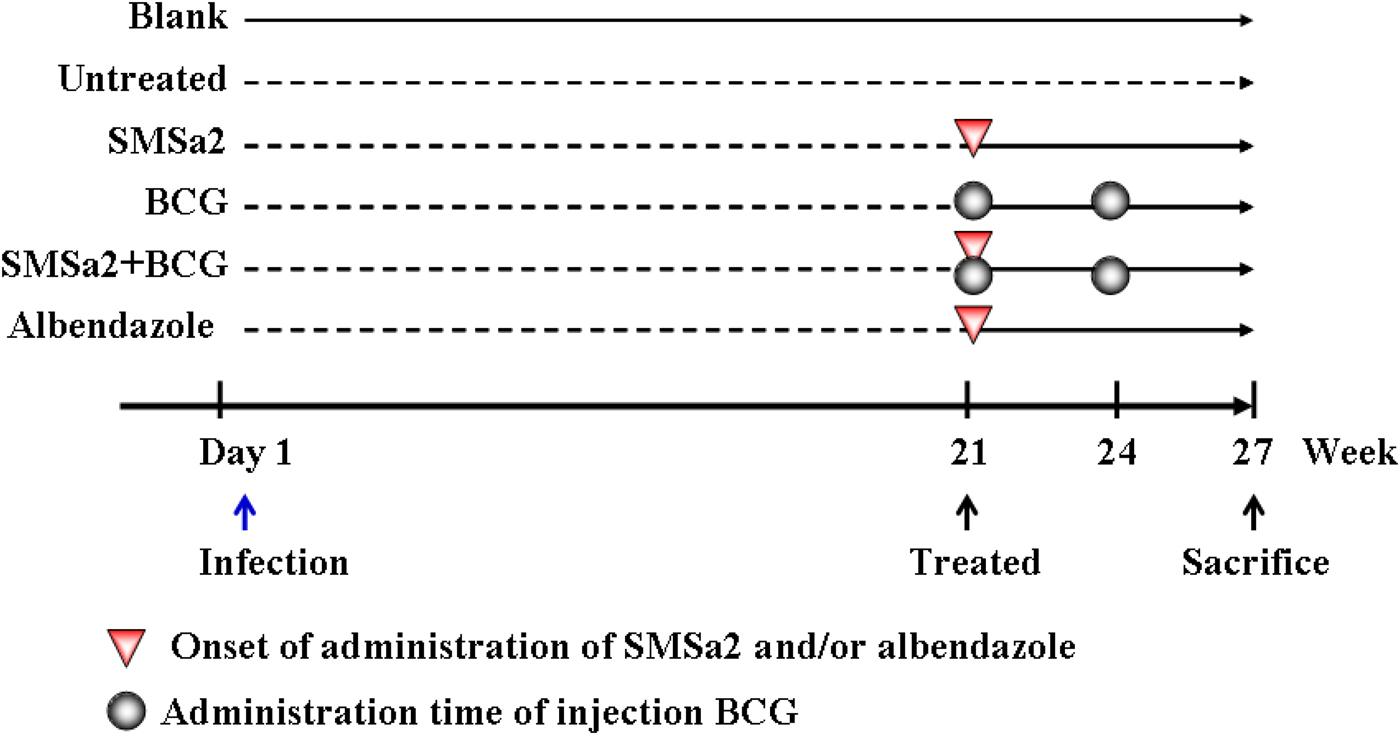
Fig. 1. Experimental procedure. After 20 weeks of infection with protoscoleces, the mice received the following treatments: (1) ABZ group: the animals were treated with a suspension of 100 mg/kg ABZ by daily intragastric administration; (2) SMSa2 group: the animals were treated with a suspension of 100 mg/kg SMSa2 by daily intragastric administration; (3) BCG group: the animals received an abdominal subcutaneous injection of 5 × 106 CFU BCG at weeks 21 and 24; (4) SMSa2 + BCG group: the animals were treated with SMSa2 and BCG as described above; and (5) untreated group and blank (uninfected) group: the animals received saline by intragastric administration as a placebo.
Assessment of cyst development
After treatment for 6 weeks, at week 27, the mice were anaesthetized with pentobarbital sodium. The hydatid cysts were harvested from the peritoneal cavity. The weight of the hydatid cysts was measured immediately to examine larval growth, and the inhibition rate was calculated as described in the literature (Yuan et al., Reference Yuan, Luo, Xin, Gao, Zhang and Jing2016).
Measurement of serum levels of IL-4 and IFN-γ
Blood samples were collected before animal euthanasia. ELISA was performed to determine the level of interleukin (IL)-4 and interferon (IFN)-γ in serum. The studied cytokines were immobilized by adsorption to the surface of 96-well plates, and primary and secondary enzyme-labelled antibodies were added to the plates. Then an appropriate substrate was added and produced a detectable product in the enzymatic reaction (Ma et al., Reference Ma, Bao, Wan, Liao, Yin, Meng, Zhou, Lu and Li2007). The colour of the solutions was read at an optical density of 450 nm immediately after the enzymatic reaction to quantify the level of cytokines in the study groups. The cytokine levels were presented as absorption units.
Staining with haematoxylin and eosin (H&E)
Specimens of hydatid cysts, liver and kidney tissues (5 × 5 mm) were cut for histological analysis. The tissue sections were fixed in Bouin solution for 24 h. All samples were dehydrated with ascending concentrations of ethanol and embedded in paraffin wax after fixation. Histology slides were stained with H&E and analysed by light microscopy.
Statistical analysis
All data are expressed as means ± SD. Statistical significance was determined by one-way analysis of variance (ANOVA) (LSD test) using the software SPSS version 17.0 (SPSS Inc., Chicago, Illinois, USA). A P value of less than 0.05 was considered to be statistically significant.
Results
SMSa2 + BCG treatment reduced cyst burden
None of the evaluated mice died in the study period, indicating no severe side-effects of the drugs used. The mean body weight in the four groups was not significantly different from that of the controls (P > 0.05, data not shown). The efficacy was determined in vivo by measuring the wet weights of hydatid cysts in infected mice. The hydatid cyst weight was significantly decreased by SMSa2 treatment (8.4 ± 3.3 g) and BCG treatment (8.3 ± 2.5 g) alone, compared with untreated mice (11.3 ± 2.1 g), although the treatment efficacy of either SMSa2 or BCG alone was not as high as that of ABZ (3.2 ± 1.2 g) at the same dose (ANOVA: df = 4, F = 14.1, P = 0.0001). However, treatment with SMSa2 + BCG (2.7 ± 1.3 g) showed an additive effect in decreasing the weight of hydatid cysts. The inhibition rate of hydatid cysts was 71.7%, 25.7%, 26.6% and 76.1% in the ABZ, SMSa2, BCG and SMSa2 + BCG groups, respectively (fig. 2). The volume of cysts in the peritoneal cavity of the untreated mice was larger than that in mice from the treated groups (fig. 2). The weight of hydatid cysts was significantly reduced in the SMSa2 + BCG group compared with the untreated (P = 0.0001), SMSa2 (P = 0.001) and BCG (P = 0.001) groups. However, there was no significant difference in the weight of hydatid cysts between the SMSa2 and BCG (P > 0.05) groups and between the ABZ and SMSa2 +BCG (P > 0.05) groups.
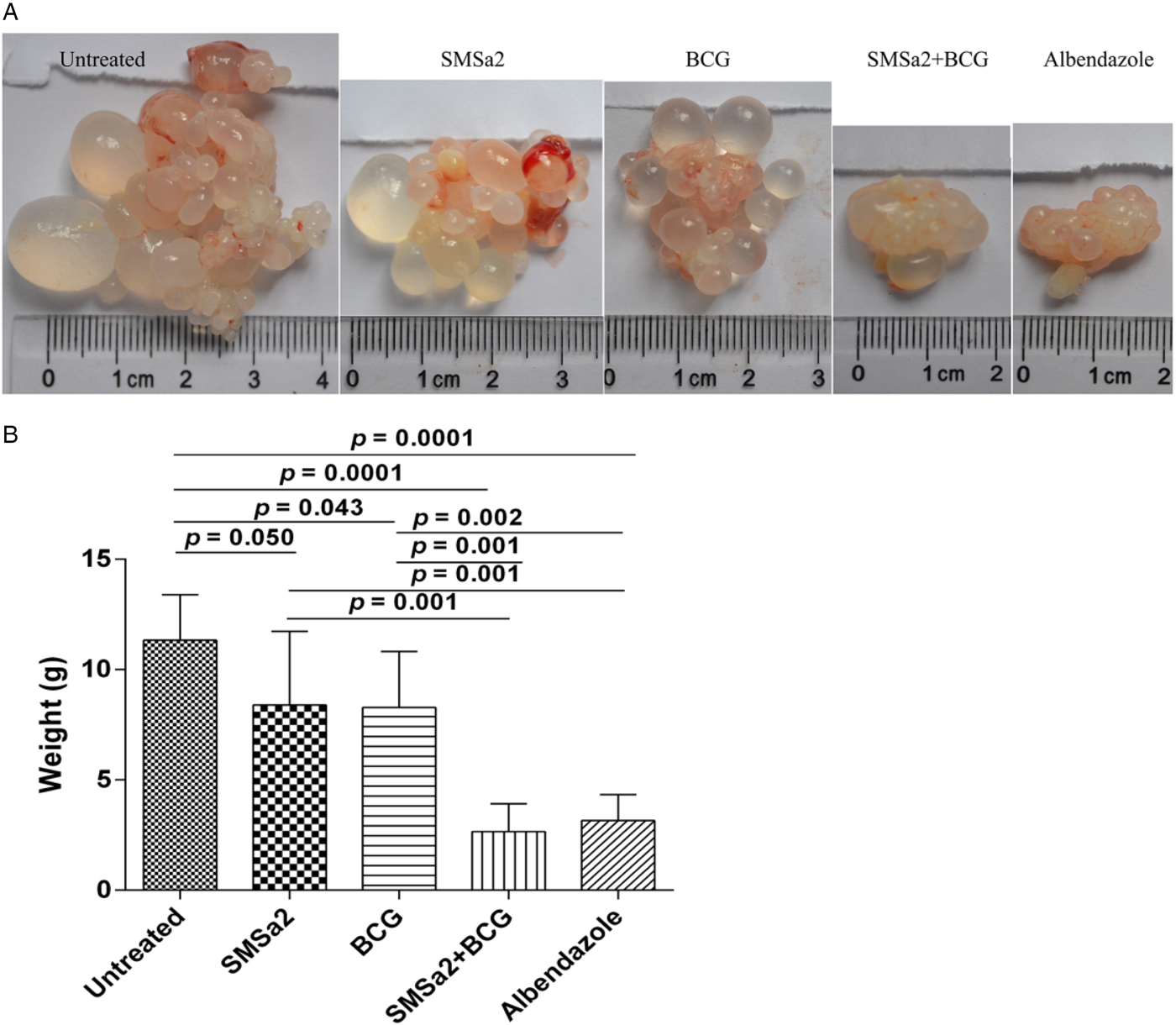
Fig. 2. The appearance (A) and weight (B) of hydatid cysts from each treatment group. Treatments were performed for 6 weeks. After euthanasia, the metacestodes were resected and weighed. Data are presented as the means ± SD, n = 5. One-way ANOVA (df = 4, F = 14.1, P = 0.0001) with the LDS test for multiple comparisons were performed for data analysis.
The cysts in the SMSa2 + BCG-treated group showed more structural modifications
The wall tissues of hydatid cysts in the untreated mice were examined by microscopy. The texture of the stratum corneum was regular and the cells of the germinal membrane were arranged in close proximity to each other (fig. 3). In contrast, the stratum corneum of hydatid cysts in mice after 6 weeks of treatment with SMSa2 + BCG was thin and the germinal membrane was degenerated and obviously ruptured. The cells of the germinal membrane of hydatid cysts in mice treated with SMSa2 + BCG were condensed and disintegrated. Moreover, conspicuous cavitations were observed in hydatid cysts in mice treated with SMSa2 + BCG (fig. 3). Taken together, the structure of the cysts in SMSa2 + BCG-treated mice was severely changed and showed morphological heterogeneity.

Fig. 3. H&E-stained images of hydatid cyst tissues (×100). Hydatid cyst tissues of all groups, showing that the cyst is surrounded by a dense inflammatory cellular infiltrate. In mice treated with albendazole or combined treatment with SMSa2 + BCG the internal tissue of hydatid cysts is completely altered, with the presence of numerous vacuoles.
The SMSa2 + BCG-treated group did not show liver and kidney tissue damage
The toxicity of SMSa2 + BCG was assessed by morphological observation in vivo. H&E-stained images of liver and kidney tissues revealed that there was no tissue damage, tearing, ballooning or neutrophil infiltration. These results indicated that liver and kidney tissues were not damaged in the treated groups.
IFN-γ and IL-4 levels in serum
Serum cytokines were quantified by ELISA (fig. 4). Compared with untreated mice (1.1 ± 1.5 pg/ml), the serum IL-4 levels were increased in mice treated with ABZ (5.7 ± 7.1 pg/ml), SMSa2 (4.2 ± 3.5 pg/ml), BCG (4.5 ± 4.0 g/ml) and SMSa2 + BCG (2.2 ± 1.6 pg/ml). However, there was no significant difference between the groups (ANOVA: df = 5, F = 1.2, p = 0.348). The serum concentration of IFN-γ in the ABZ, SMSa2, BCG, SMSa2 + BCG and untreated groups was 16.8 ± 15.4 pg/ml, 8.7 ± 7.9 pg/ml, 8.5 ± 6.7 pg/ml, 1.0 ± 0.8 pg/ml and 7.0 ± 3.9 pg/ml, respectively (fig. 4). The serum IFN-γ levels were non-significantly decreased in SMSa2 + BCG-treated mice compared with untreated mice (ANOVA: df = 5, F = 2.1, P = 0.101).

Fig. 4. The serum IL-4 (A) and INF-γ (B) levels in mice of each group. Data are presented as the means ± SD, n = 5. One-way ANOVA with LDS test for multiple comparisons were performed for data analysis (ANOVA of IL-4 data: df = 5, F = 1.2, P = 0.348; ANOVA of INF-γ data: df = 5, F = 2.1, P = 0.101).
Discussion
CE is a near-cosmopolitan zoonotic parasitic disease and is widespread in western China. This disease can occur as a result of infection with E. granolusus (Ma et al., Reference Ma, Bao, Wan, Liao, Yin, Meng, Zhou, Lu and Li2007). Appropriate therapy for CE should be implemented considering different factors, including patient age, cyst location and size, and structural characterization of the lesions (Jelowdar et al., Reference Jelowdar, Rafiei, Abbaspour, Rashidi and Rahdar2017). However, an effective and safe treatment for CE is a research priority. We have previously extracted a lipophilic fraction of alkaloids from S. moorcroftiana seeds and have shown that this fraction has a weak inhibitory effect on CE in mice (Ma et al., Reference Ma, Li, Wang and Yin2005). The present study was the first to demonstrate that the combined therapy of SMSa2 and BCG was effective against CE.
The mouse model of infection was established by inoculating 5000 E. granulosus protoscolices intraperitoneally. After 20 weeks of infection, the mice were treated with SMSa2, BCG or SMSa2 + BCG for 6 weeks. The results indicated that alkaloids and BCG alone significantly decreased the hydatid cyst weight, although the efficacy was not as high as that of ABZ at the same dose. However, SMSa2 + BCG showed an additive effect and reduced the hydatid cyst weight significantly. The inhibition rate of hydatid cysts in the combined SMSa2 + BCG treatment was 76.1% and significantly higher than that of mice treated with the ABZ, alkaloids or BCG alone. Moreover, the stratum corneum of hydatid cysts in mice treated with SMSa2 + BCG was thin, and the germinal membrane was degenerated and ruptured, and these features may be considered indirect effects of treatment. Optical microscopy showed that there was no cell damage in liver and kidney tissues from mice treated with alkaloids and BCG, and confirmed that SMSa2 + BCG was efficient against echinococcosis.
Cytokines may play an important role in the host response to E. granulosus infection. The detection of serum levels of IL-4 and IFN-γ in vivo has been suggested to be helpful in monitoring the therapeutic effects of treatment (Torcal et al., Reference Torcal, Navarro-Zorraquino, Lozano, Larrad, Salinas, Ferrer, Roman and Pastor1996; Moreno et al., Reference Moreno, Urrea-Paris, Casado and Rodriguez-Caabeiro2001). In the present study, there was no marked change in the serum levels of IL-4 and IFN-γ in the treated groups. Although BCG can induce Th1-type cellular immune responses against tumours and parasites (Li et al., Reference Li, Han, Zhu, Cui, Ma and Dong2016), the results of the present study indicated that the immune response mediated by Th1 and Th2 cells was weak in late-stage E. granulosus infection. However, compared with the untreated group, the serum levels of IL-4 had a tendency to increase in all treated groups, indicating that a few Th2 cells were activated by antigens of protoscoleces to eliminate target cells after treatment. The combined treatment with SMSa2 and BCG is efficient against echinococcosis in mice infected experimentally with protoscoleces of E. granulosus. However, further studies on the mechanism of action of drugs used to treated echinococcosis are necessary for the development of effective combined therapies against this disease.
Financial support
This study was funded by the Fundamental Research Funds for the Central Universities (lzujbky-2017-117) and Lanzhou University Innovation and Entrepreneurship Fund for College Students (2015073001362).
Conflicts of interest
None.
Ethical standards
All experiments were carried out according to protocols approved by the Institutional Animal Care and Use Committee. Mice were housed in a standard animal room with food and water ad libitum. All experimental procedures involving mice were approved by the Institutional Animal Care and Use Committee of Lanzhou University.






