Introduction
Characterising the diversity and taxonomic relationships of taeniid tapeworms (Platyhelminthes: Cestoda) has been historically complicated, though of significance because of their socioeconomic impact on domestic livestock and human health (Hoberg Reference Hoberg2006; Lavikainen et al. Reference Lavikainen, Haukisalmi, Lehtinen, Henttonen, Oksanen and Meri2008). Recent research using molecular tools to organise the family Taeniidae has driven the erection of the genus Versteria (Nakao et al. Reference Nakao, Lavikainen, Iwaki, Haukisalmi, Konyaev, Oku, Okamoto and Ito2013), with the type species Taenia mustelae Gmelin, Reference Gmelin1790 [syn. Taenia tenuicollis (Rudolphi, Reference Rudolphi1819), Taenia brevicollis (Rudolphi, Reference Rudolphi1819), Fimbriotaenia mustelae (Gmelin, Reference Gmelin1790)] (Nakao et al. Reference Nakao, McManus, Schantz, Craig and Ito2007; Lavikainen et al. Reference Lavikainen, Haukisalmi, Lehtinen, Henttonen, Oksanen and Meri2008; Knapp et al. Reference Knapp, Nakao, Yanagida, Okamoto, Saarma, Lavikainen and Ito2011; Nakao et al. Reference Nakao, Lavikainen, Iwaki, Haukisalmi, Konyaev, Oku, Okamoto and Ito2013). This genus remains poorly characterised, with only three nominal species described (Bagnato et al. Reference Bagnato, Gilardoni, Martin and Digiani2022); at the time of its erection, only two species were included, V. mustelae and V. brachyacantha (Baer and Fain Reference Baer and Fain1951), the latter based solely on morphological similarities as no sequences were available (Nakao et al. Reference Nakao, Lavikainen, Iwaki, Haukisalmi, Konyaev, Oku, Okamoto and Ito2013). This separation was supported by previous studies, which noted that V. mustelae was basal in Taenia based on morphometric diagnostic characteristics such as its minute rostellar hooks, small scolex, rostellum, and suckers, a limited number of testes, and relatively short strobila (Hoberg et al. Reference Hoberg, Jones, Rausch, Eom and Gardiner2000). It was these unique characteristics that had previously defined the assignment of specimens to V. mustelae, the species something of a catch-all for the small-hooked taeniids of mustelids (Freeman Reference Freeman1956; Nakao et al. Reference Nakao, Lavikainen, Iwaki, Haukisalmi, Konyaev, Oku, Okamoto and Ito2013). This species was redescribed numerous times in the 20th century; large variances in the range of diagnostically important characteristics were reported between North America, Europe, and Asia (Table 1) (Thienemann Reference Thienemann1906; Skinker Reference Skinker1935; Locker Reference Locker1955; Freeman Reference Freeman1956; Abuladze Reference Abuladze1964; Wahl Reference Wahl1967; Verster Reference Verster1969; Iwaki et al. Reference Iwaki, Abe, Shibahara, Oku and Kamiya1995). Nakao et al. (Reference Nakao, Lavikainen, Iwaki, Haukisalmi, Konyaev, Oku, Okamoto and Ito2013) noted that differences in the cysticerci of Palearctic and Nearctic V. mustelae might suggest cryptic species. Lavikainen et al. (Reference Lavikainen, Haukisalmi, Lehtinen, Henttonen, Oksanen and Meri2008) reported little genetic differentiation among Palearctic specimens, while no analysis was made between Nearctic examples.
Table 1. Comparison of measurements for Versteria. Morphometric ranges for diagnostic characteristics for other species of Versteria synthesized and compared against those taken for V. rafei n. sp. from this study. Measurements given in micrometers. Reports that did not differentiate long hooks from short hooks and only provided a general measurement are presented as a single range. Abbreviations: LH, large hooks; SH, small hooks; L, length; W, width

Molecular characterisation of zoonotic infections and recent morphological analysis of specimens suggest additional taxa related to Versteria are present in North America. Recent reports of echinococcus-like infections have identified taeniid cysticerci in human patients from Pennsylvania (USA) and New Brunswick (Canada) (Barkati et al. Reference Barkati, Gottstein, Müller, Sheitoyan-Pesant, Metrakos, Chen, Garceau, Libman, Ndao and Yansouni2019; Lehman et al. Reference Lehman, Leal, Procop, O’Connell, Shaik, Nash, Nutman, Jones, Braunthal, Shah, Cruise, Mukhopadhyay and Banzon2019) as well as in an orangutan Pongo pygmaeus at the Milwaukee County Zoo, Wisconsin, that was born in Colorado (USA) (Lee et al. Reference Lee, Wallace, Clyde, Gendron-Fitzpatrick, Sibley, Stuchin, Lauck, O’Connor, Nakao, Lavikainen, Hoberg and Goldberg2016; Deplazes et al. Reference Deplazes, Eichenberger and Grimm2019). Molecular analysis of these infections identified the parasite as belonging to Versteria, and molecular characterisation suggested that there may be two or more Versteria species in the Nearctic (Lee et al. Reference Lee, Wallace, Clyde, Gendron-Fitzpatrick, Sibley, Stuchin, Lauck, O’Connor, Nakao, Lavikainen, Hoberg and Goldberg2016; Niedringhaus et al. Reference Niedringhaus, Ganoe, Lovallo, Walter, Yabsley and Brown2022). Because of this, and due to the wide variation in reported morphological characteristics between North American and European samples, previous identifications of V. mustelae from North America may have been incorrect. Lee et al. (Reference Lee, Wallace, Clyde, Gendron-Fitzpatrick, Sibley, Stuchin, Lauck, O’Connor, Nakao, Lavikainen, Hoberg and Goldberg2016) investigated mustelids in Oregon, Colorado, and Wisconsin to identify adults of the unnamed species, producing a single specimen each from a mink Neogale vison in Oregon, an ermine Mustela erminea in Wisconsin, and an ermine in Colorado. Unfortunately, the samples were fragmented, were without scoleces, and could not be fully described. Molecular analysis confirmed the specimens from Oregon and Colorado to be the same lineage as those identified from the orangutan infection, while the specimen from Wisconsin clustered closer to haplotypes from the Palearctic confirming there are likely multiple species present in North America (Lee et al. Reference Lee, Wallace, Clyde, Gendron-Fitzpatrick, Sibley, Stuchin, Lauck, O’Connor, Nakao, Lavikainen, Hoberg and Goldberg2016). Recently, a third species was added, Versteria cuja Bagnato, Gilardoni, Martin & Digiani, Reference Bagnato, Gilardoni, Martin and Digiani2022, identified from the lesser grison Galictis cuja in Argentina and molecularly distinct from the Nearctic haplotypes available (Bagnato et al. Reference Bagnato, Gilardoni, Martin and Digiani2022).
Previous studies of parasites identified as V. mustelae, which likely included two or more species in North America, have reported various hosts. Definitive hosts included ermine, mink, and marten Martes americana (Skinker Reference Skinker1935; Locker Reference Locker1955; Freeman Reference Freeman1956; Miller and Harkema Reference Miller and Harkema1964; Jennings et al. Reference Jennings, Threlfall and Dodds1982). Intermediate hosts included multiple species of terrestrial rodents, including members of Microtus and Peromyscus, fox squirrels Sciurus niger in Michigan, and muskrats Ondatra zibethicus in Alaska, British Columbia, and Illinois (Skinker Reference Skinker1935; Locker Reference Locker1955; Langham et al. Reference Langham, Rausch and Williams1990). A fatal infection of Versteria sp. in a muskrat from Pennsylvania was sequenced. It clustered with infections from a human in Pennsylvania, the orangutan mentioned above, and adult parasite specimens from Oregon and Colorado (Niedringhaus et al. Reference Niedringhaus, Ganoe, Lovallo, Walter, Yabsley and Brown2022). This zoonotic infection by Versteria sp. is cause for concern and identification of the adults and their hosts is essential in the mitigation of infection in humans.
In this article, we describe this unnamed species as Versteria rafei n. sp. from the intestines of Neogale vison based on morphological and molecular data. We report infections identified in N. vison, Lontra canadensis, and Ondatra zibethicus and larval infections in the livers of N. vison and O. zibethicus from British Columbia and Alberta, Canada.
Materials and methods
Carcasses of L. canadensis (n=155) and N. vison (n=106) were obtained from licensed fur trappers in Alberta and British Columbia (BC), Canada, during the 2020–21 and 2021–22 trapping seasons. Muskrats O. zibethicus (n=41) were only collected during the 2020–21 trapping season from Alberta. Carcasses were frozen after skinning and kept at -20°C, except during shipping or transport, until necropsy. Cestodes were collected during necropsy and preserved and stored in 70% ethanol or 90% ethanol for molecular analysis. For morphological analysis, specimens were stained with acetic acid carmine, dehydrated in a graduated ethanol series, cleared in clove oil, and mounted permanently in Canada balsam for examination by light microscope. Photomicrographs were taken on an Olympus BX51 compound microscope mounted with DP25 camera attachment (Olympus, Tokyo), which calculated scale and included a scale bar in the picture. Measurements of various morphological features were then taken using the software ImageJ (Schneider et al. Reference Schneider, Rasband and Eliceiri2012). Measurements are given in μm unless otherwise stated, with average and standard deviation in parentheses.
Molecular analysis
Molecular identification was carried out based on the nad1 mitochondrial region (Forward NDJ11: 5’-AGATTCGTAAGGGGCCTAATA-3’, Reverse NDJ2a: 5’-ACCACTAACTAATTCACTTTC-3’) and the cox1 mitochondrial region (Forward JB3: 5’-5TTTTTTGGGCATCCTGAGGTTTAT-3’, Reverse JB4.5: 5’-TAAAGAAAGAACATAATGAAAATG-3’) using primers suggested for taeniids following the suggested cycling instructions of the authors (Bowles and McManus Reference Bowles and McManus1993; Bowles et al. Reference Bowles, Blair and McManus1994; Trachsel et al. Reference Trachsel, Deplazes and Mathis2007). DNA was extracted from terminal proglottids using DNeasy Blood & Tissue Kit (QIAGEN, Hilden, Germany) using manufacturer’s protocols. Amplification and sequencing were conducted with a total volume of 25μl using PCR Master Mix (Invitrogen) according to the manufacturer’s instructions. PCR products were cleaned using EXOSAPTM Express PCR Product Cleanup Reagent (USB Corporation, Cleveland, OH, USA) following manufacturer’s instructions. Sanger sequencing was performed by the University of Alberta’s Molecular Biology Service Unit (Edmonton, Canada) or by the Genetic Analysis Service, Department of Anatomy, University of Otago (Dunedin, New Zealand). The produced sequences were trimmed and compared against those available in GenBank using BLAST (https://blast.ncbi.nlm.nih.gov/Blast.cgi). Exemplar sequences have been deposited in GenBank.
Phylogenetic analysis
Sequences were aligned using ClustalW as implemented in MEGA11 software (Kumar et al. Reference Kumar, Stecher, Li, Knyaz and Tamura2018) against sequences from related parasites in GenBank. The resulting alignments were used to create a phylogenetic tree by Maximum-likelihood method and the Tamura-Nei model (Tamura Reference Tamura1992; Tamura and Nei Reference Tamura and Nei1993) bootstrapped at 500 replicates. Estimates of evolutionary divergence were quantified by calculating pairwise distance conducted in MEGA11 (Tamura et al. Reference Tamura, Stecher and Kumar2021). The analyses involved 8 (nad1) and 12 (cox1) nucleotide sequences. Codon positions included were 1st+2nd+3rd+Noncoding. There was a total of 452 (nad1) and 347 (cox1) positions in the final dataset.
Results
Adult cestodes identified as Versteria sp. were found in all three species (nmink = 8 [7.5%]; notter= 5 [3.2%]; nmuskrat = 2 [4.9%]). Larval infections were identified in the livers of mink and muskrat in Alberta (nmink = 4 [3.7%]; nmuskrat= 18 [43.9%]), at intensities ranging from 3 cysts to over 50. The mink infected with over 50 cysts in the liver was also notable for concurrently being infected with 21 adults.
Description of new species
Family. Taeniidae Ludwig, 1886
Genus. Versteria Nakao, Lavikainen, Iwaki, Haukisalmi, Konyaev, Oku, Okamoto, & Ito, Reference Nakao, Lavikainen, Iwaki, Haukisalmi, Konyaev, Oku, Okamoto and Ito2013
Species. Versteria rafei n. sp.
Material studied. Ten complete adults and strobilar fragments with and without scoleces from 10–20 adults, which included immature, mature, and gravid proglottids.
Description. Strobila short, complete gravid specimens ranging from 200 to over 1000 mm (Figure 1). Scolex small, 170–220 (188±16 SD) wide, with rostellum 39–67 (54±9 SD) in diameter at widest point (n=14). Suckers large in relation to scolex, 81–101 (90±6 SD) wide (Figure 2). Neck tapering immediately posterior to suckers, 88–120 (100±9 SD) wide (n=14). Rostellum with double crown of very small hooks, ranging in number from 21–24 per row (42–48 total) (n=5); scoleces almost always found without hooks, or with only a few hooks remaining. Hooks with sharp curved blade, long and stout guard with bulbous epiphyseal thickening, and a short or long, straight handle with narrow epiphyseal thickening; hooks in posterior circle with short handle, thick base, and thorn-like shape, in anterior circle with long handle and thin base (Figure 2E, F). Little difference in length between the short and long handled hooks, short 10–15 (12.2±1.6 SD) (n=25) and long 13–17 in length (14.9±1.4 SD) (n=25). Proglottids craspedote: immature proglottids broader than long, mature and gravid proglottids longer than wide. Mature proglottids with length/width ratio of 1:1.07–1.91 (1:1.51±0.22 SD), gravid proglottids with length/width ratio of 1:1.03–4.37 (1:1.97±0.70 SD). Mature proglottids 629–1323 (904±152 SD) long and 384–1003 (610±120 SD) wide (n=71). Gravid proglottids 1203–3489 (1860±582 SD) long, 726–1241 (979±187 SD) wide; 18–28 uterine branches (n=30). Genital pores alternate irregularly, usually slightly anterior of middle of proglottid, protruding in older proglottids. Genital atrium well developed, with muscular sphincter, rounder than oval when relaxed, forming big genital papilla (Figure 2B); in mature proglottids 57–162 (122±24 SD) deep and 52–141 (95±586 SD) wide when relaxed (n=71); in gravid proglottids, 97–201 (146±29 SD) deep and 65–180 (130±25 SD) in width when relaxed (n=30). Longitudinal osmoregulatory canals 28 (±7 SD) wide (n=14); transverse connecting canals 18 (±4 SD) wide (n=14) in mature proglottids.
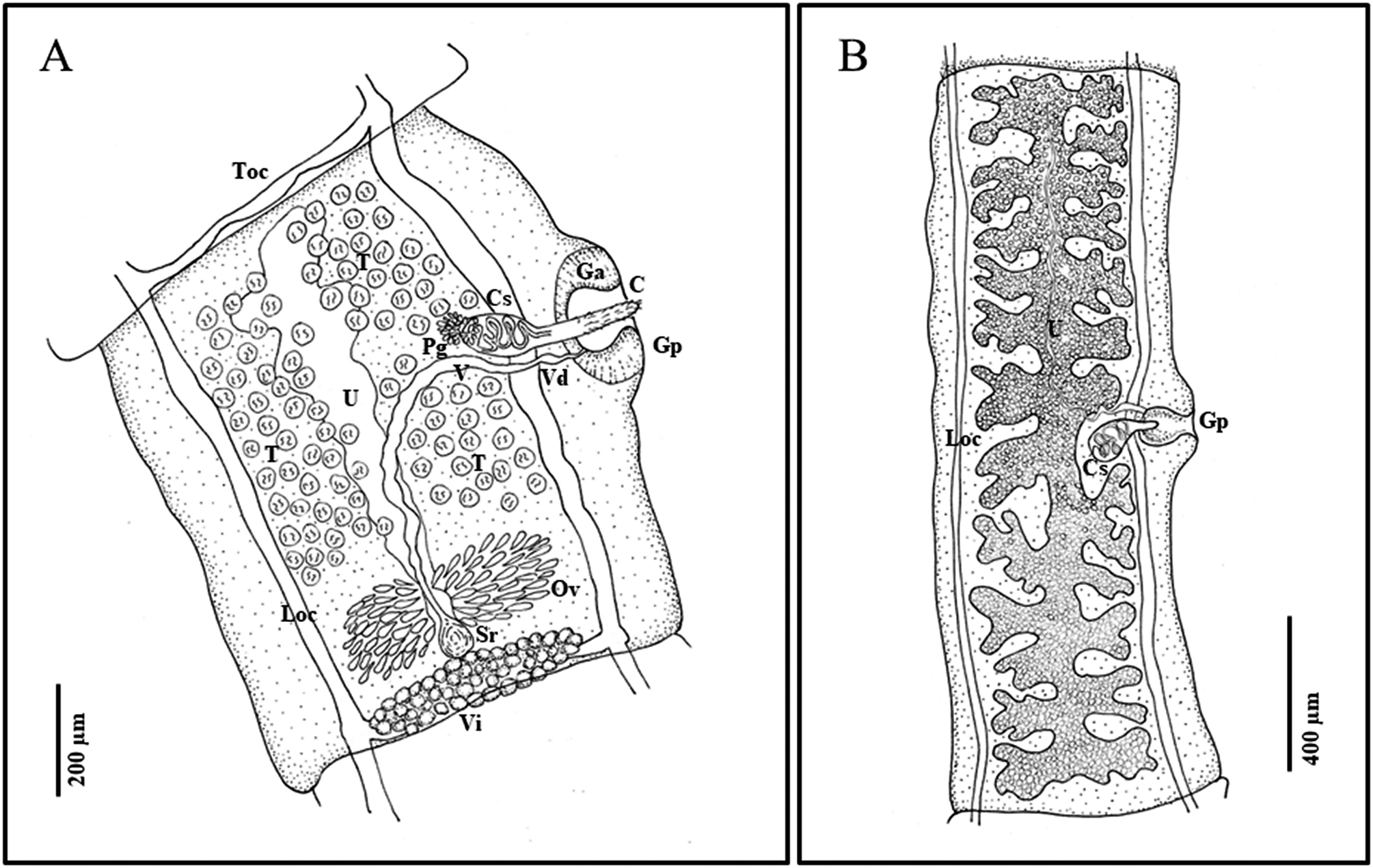
Figure 1. Line drawings of Versteria rafei n. sp. (Cestoda: Taeniidae). Examples from the North American Mink (Neogale vison) from British Columbia, Canada. A) mature proglottid; B) gravid proglottid. Abbreviations: C, cirrus; Ci, cirrus sac; Ga, genital atrium; Gp, genital pore; Loc, longitudinal osmoregulatory canal; Ov, ovary; Pg, prostatic gland; Sr, seminal receptable; T, testes; Toc, transverse osmoregulatory canal; U, uterus; V, vagina; Vd, vas deferens; Vi, vitellarium. Illustrated by B. Presswell.

Figure 2. Specimens of Versteria rafei n sp. Examples from the North American mink (Neogale vison) from British Columbia, Canada. A) mature proglottid of an adult worm, stained with acetic acid carmine, cleared in clove oil, and mounted permanently in Canada balsam; B) close-up of cirrus sac and genital pore; box from panel A; C) partially everted cirrus of adult showing bristles; D) scanning electron microscope image of scolex (missing hooks), showing the rostellum and suckers, magnification at 995x; E) double crown of rostellar hooks, prepared by staining in acetic acid carmine and then squashing the scolex on a glass slide with a cover slip and rotating, with close-ups of hooks with short and long guards; F) line drawing of hooks. Abbreviations: b, blade; C, cirrus; Ci, cirrus sac; g, guard; Ga, genital atrium; Gp, genital pore; h, handle; Loc, longitudinal osmoregulatory canal; N, neck; Ov, ovary; Pg, prostatic gland; R, rostellum; S, sucker; T, testes; U, uterus; V, vagina; Vi, vitellarium.
Male reproductive system. Testes 85–117 in number (n=71), generally sub-spherical in shape, 16–36 (23±4 SD) in diameter (n=131). Testicular fields confluent anteriorly, situated between longitudinal osmoregulatory canals, from anterior margin of proglottid to anterior margin of ovary. Antero-poral field with fewest testes; antero-poral and postero-poral fields interrupted by vagina and cirrus sac (Figure 1). Cirrus sac ovoid in shape and relatively small, 105–195 (150±21 SD) long and 46–113 (82±29 SD) wide in mature proglottids (n=71), and 145–242 (183±22 SD) long and 87–139 (109±15 SD) wide in gravid proglottids (n=30). Vas deferens forming loops inside and outside cirrus sac, surrounded by prostatic cells. Cirrus armed with hair-like bristles in a spiral pattern.
Female reproductive system. Ovary two-winged, wings roughly equal in size, lobed. Shape and size vary depending on stage of development; in mature proglottids at posterior, 248–390 (293±62 SD) wide (n=10); in mature to gravid proglottids, round and strongly staining, often just posterior of center (Figure 2). Vitellarium just posterior of ovary and seminal receptacle, at posterior edge of proglottid, averaging 337 (±34 SD) wide; proglottid width to ovary width ratio 1:0.29–0.49 (1:0.41±0.08 SD) (n=10). Vagina wide, enters genital pore posteriorly opening of cirrus sac and directed centrally and posteriorly before connecting with seminal receptacle. Uterus fills medium portion of proglottid, with outpocketings at irregular intervals along its length, largest at anterior end of uterus (Figure 1). Gravid proglottids with 18–28 total lateral branches (n=30), often with secondary and sometimes tertiary bifurcations. Eggs (embryophores) sub-ovoid, 18–22 in length and 14–18 in diameter (n=9).
Taxonomic summary
Type host: Neogale vison (Carnivora: Mustelidae).
Other hosts: definitive, Lontra canadensis (Carnivora: Mustelidae), Ondatra zibethicus (Rodentia: Cricetidae); intermediate N. vison, O. zibethicus.
Type locality: Southeast Vancouver Island, British Columbia, Canada.
Known distribution: Western Canada: Alberta and British Columbia.
Site of infection: Small intestine (definitive host), liver (intermediate host).
Prevalence and intensity of infection: N. vison: 7.5% (n=106), intensity 5–54 per host; L. canadensis: 3.2% (n=155), intensity 3–23 per host; O. zibethicus: 4.9% (n=41), intensity 2–4 per host. Larval infections in the liver, N. vison: 3.8% (n=106); O. zibethicus: 43.9% (n=41).
Type specimens: Syntypes, CMNPA 2023-0008.1, CMNPA 2023-0008.2, CMNPA 2023-0008.3, Canadian Museum of Nature, Ottawa.
Etymology: The specific epithet (a noun in the genitive case) honours Dr. Rafael ‘Rafe’ R. Payne, a parasitologist who dedicated almost 50 years of his life to the education of undergraduate students.
GenBank accession numbers: OR448764 (cox1, adult, N. vison), OR852790 (nad1, adult, N. vison), OR852791 (nad1, metacestode, N. vison), OR863684 (nad1, adult, L. canadensis), OR852792 (nad1, adult, O. zibethicus), OR852793 (nad1, metacestode, O. zibethicus).
ZooBank access number: DBD28DD4-DBB4-4931-92A5-F5D0BD897510
Remarks: According to molecular evidence and the diagnosis given by Nakao et al. (Reference Nakao, Lavikainen, Iwaki, Haukisalmi, Konyaev, Oku, Okamoto and Ito2013), Versteria rafei n. sp. belongs to the genus Versteria due to its short strobila, elongate gravid proglottids, small scolex, rostellum, suckers, and double crown of very small hooks. Other characteristics of the genus include genital pores that alternate irregularly roughly at the middle of the proglottid and terminal genital ducts that pass longitudinally over the osmoregulatory canals, median, posterior female glands with bilobed ovaries, transversely elongated vitellarium posterior to the ovary, median uterus a longitudinal stem, laterally branched when gravid, and relatively small number of testes almost entirely anterior and lateral to the female organs. It differs from the diagnosis only in that its mature proglottids are not wider than they are long. Mature proglottids are generally close to equal in length and width, though more often longer than they are wide. Descriptions of V. mustelae, V. brachyacantha, and V. cuja all reported mature proglottids wider than long (Table 1). However, the recent description of V. cuja also reported ranges for length and width that overlap in mature proglottids (476–2027 long by 1117–1941 wide) and might have included proglottids that were longer than wide. In fact, their published line drawing showed a mature proglottid longer than wide (Bagnato et al. Reference Bagnato, Gilardoni, Martin and Digiani2022). Skinker (Reference Skinker1935) noted that mature proglottids were generally wider than long, but that the ratio also varied. Descriptions of V. mustelae by Wahl (Reference Wahl1967) and Joyeux and Baer (Reference Joyeux and Baer1936) did not provide the length-width ratio for mature proglottids. In the description by Freeman (Reference Freeman1956) of natural and experimental infections in weasel and mink from North America, the author reported a length–width ratio of 1:0.54–1.71 in weasel Mustela nivalis and 1:1.63–1.22 in mink, showing a range of ratios in weasels, while all examples reported from mink were longer than wide. It is likely that this ratio is dependent on the age of the mature proglottid, wider when young as it transitions from an immature stage but then gradually longer than wide as it matures. This ratio may also be host-dependent as Freeman (Reference Freeman1956) has reported ratios in mink that were similar to those we report from mink here. We suggest the diagnosis for Versteria be amended to state that immature proglottids are wider than long, gravid proglottids longer than wide, and that mature proglottids vary depending on their level of maturity and possibly host.
Since historically V. mustelae was a catch-all species for the small-hooked taeniids of mustelids, and morphological descriptions vary widely over the years and between North America and Eurasia (Table 1), it is difficult to accurately compare the species described herein to the rest of the genus. Modern molecular analysis has identified one or more distinct lineages of Versteria in North America, and others may exist in Eurasia (Lee et al. Reference Lee, Wallace, Clyde, Gendron-Fitzpatrick, Sibley, Stuchin, Lauck, O’Connor, Nakao, Lavikainen, Hoberg and Goldberg2016; Niedringhaus et al. Reference Niedringhaus, Ganoe, Lovallo, Walter, Yabsley and Brown2022), which may explain the large variation in morphological characteristics previously reported for V. mustelae. However, there are several distinct features that characterise V. rafei n. sp. from the three other species of Versteria.
For the main diagnostic characteristics of the scolex, rostellum, suckers, hook size and number, cirrus sac, genital atrium, and uterine branches, V. rafei n. sp. is distinct in the size of its scolex and sucker to scolex ratio, the size of its cirrus sac, and the depth of its genital atrium. It has the smallest scolex of all Versteria species (170–220 μm), with large suckers compared to the scolex (0.46–0.48 of the scolex width). In comparison, V. cuja in South America (276–345 μm, 1:0.32–0.49) (Bagnato et al. Reference Bagnato, Gilardoni, Martin and Digiani2022), V. brachyacantha in Central Africa (480 μm, 1:0.37) (Baer and Fain Reference Baer and Fain1951), and species reported as V. mustelae from Europe (260–550 μm, 1:0.24–0.50) (Thienemann Reference Thienemann1906; Abuladze Reference Abuladze1964; Wahl Reference Wahl1967) have much larger scoleces. It also has the smallest cirrus sac (105–176 μm long, 46–95 μm wide) and ovoid in shape compared to the more spherical V. cuja (210–311 μm long, 130–185 μm wide) (Bagnato et al. Reference Bagnato, Gilardoni, Martin and Digiani2022), V. brachyacantha (240–280 μm long, 120 μm wide) (Baer and Fain Reference Baer and Fain1951), and V. mustelae in Europe (229–369 μm long, 123–176 μm wide) (Abuladze Reference Abuladze1964; Wahl Reference Wahl1967). The genital atrium (105–157 μm) is deeper than that reported in V. mustelae in Europe (68–91 μm) (Wahl Reference Wahl1967), but smaller than V. cuja (170–420 μm) (Bagnato et al. Reference Bagnato, Gilardoni, Martin and Digiani2022). Note that there is no report of the atrium depth for V. brachyacantha. However, the published line drawing shows the genital atrium as equal to or slightly larger than the length of the cirrus sac (240–280 μm) (Baer and Fain Reference Baer and Fain1951).
Besides these diagnostic characteristics, what distinguishes V. rafei n. sp. from V. mustelae and V. cuja is its armed cirrus (Iwaki et al. Reference Iwaki, Abe, Shibahara, Oku and Kamiya1995; Bagnato et al. Reference Bagnato, Gilardoni, Martin and Digiani2022), which has hair-like bristles in a spiral pattern, similar to V. brachyacantha (Baer and Fain Reference Baer and Fain1951). The rostellar hooks of V. rafei n. sp. (10–17 μm) are similar in size to those of V. cuja (12–17 μm), but they are different in shape. Versteria cuja only has hooks with long handles and a short blade (Bagnato et al. Reference Bagnato, Gilardoni, Martin and Digiani2022), similar to V. mustelae, which also has a short blade and no difference in handle size (Verster Reference Verster1969). They are much smaller than the hooks of V. brachyacantha (26–28 μm, c.54) with fewer hooks, though there is some similarity in shape with an enlarged flattened guard, long thorn-like blade, and smallish handle even in the long-handled specimens (Baer and Fain Reference Baer and Fain1951).
Metacestodes
Cysts containing cysterceri were identified in the liver of muskrats and mink (nmuskrat= 18 [43.9%]; nmink = 4 [3.7%]), ranging in intensity from 1 to over 50 cysts. They were found in the parenchyma of the liver, most often easily seen in the periphery, though at times embedded within and obscured by tissue. Cyst walls were thin and transparent, the number of larvae ranging from 6 to 18, all closely applied to the inner wall of the host cyst (Figure 3). Larvae were covered by a thin capsule, 0.75–4 mm in diameter, with round calcareous corpuscles and invaginated small scoleces, mostly uniscolex, some with up to 4 scoleces. Scoleces contained two rows of short rostellar hooks of similar size and shape as described in adult specimens.

Figure 3. Cysts of Versteria rafei n sp. Examples from muskrat (Ondatra zibethicus) from Alberta, Canada. A) two cysts in a lobe of the liver; B) excised cyst from the liver showing multiple metacestodes adhered to the cyst wall.
Molecular and phylogenetic analysis
Extraction, PCR, and sequencing produced 33 nad1 sequences from adult specimens in mink (nAB=20, nBC=6), otters (nAB=3, nBC=2), and muskrat (nAB=2), and 6 nad1 sequences from metacestodes in the livers of mink (nAB=2) and muskrat (nAB=4). A recent study described a new species, V. cuja, in South America using the cox1 mitochondrial region, so we conducted a second analysis using the cox1 region in order to compare against this new species. Three cox1 sequences were also produced from adult specimens in mink (nAB=1, nBC=2). Phylogenetic analysis of available nad1 and cox1 sequences in GenBank confirmed our study specimens to be a sister lineage to larval infections previously identified and likely represent a separate species from V. cuja; 100% of bootstrapped trees clustering the rest of the North American examples separate from V. cuja while still making a distinct North American clade (Figure 4). One worm from a river otter in Alberta produced a nad1 sequence unique from the other 28, with a pairwise distance of 0.046 from V. rafei n. sp., which is more than the distance reported between the newly described Versteria cuja and Versteria sp. in North America, and may represent another species (Bagnato et al. Reference Bagnato, Gilardoni, Martin and Digiani2022). Unfortunately, only one fragmented specimen was found, so description was not possible. However, this further supports assumptions that Versteria is likely a species complex in North America (Lee et al. Reference Lee, Wallace, Clyde, Gendron-Fitzpatrick, Sibley, Stuchin, Lauck, O’Connor, Nakao, Lavikainen, Hoberg and Goldberg2016; Bagnato et al. Reference Bagnato, Gilardoni, Martin and Digiani2022).

Figure 4. Phylogenetic trees of parasites described as Versteria rafei n. sp. Mitochondrial sequences from this study identified in red, including host species, and location of capture in brackets for the A) nad1 and B) cox1 mitochondrial regions. All sequences were from adult specimens unless noted as produced from metacestodes. Sequences are compared against sequences from GenBank, identified by ascension number before their name. Sequences identified in GenBank as Versteria sp. or Versteria mustelae that are likely examples of V. rafei are identified as V. cf. rafei. One sequence collected in this study was labelled as Versteria sp. due to increased evolutionary distance, which suggests it may be a unique species. The evolutionary history was inferred using the Neighbor-Joining method (Saitou and Nei Reference Saitou and Nei1987). The optimal tree is shown. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1000 replicates) are shown next to the branches (Felsenstein Reference Felsenstein1985). The tree is drawn to scale, with branch lengths in the same units as those of the evolutionary distances used to infer the phylogenetic tree.
Intra- and interspecific variation
Average intraspecific pairwise distance for V. rafei n. sp. was 0.007 for the nad1 gene (n=33) and 0.002 for the cox1 gene (n=3) amongst our samples. For the eight samples used in the phylogenetic analysis, examination of intraspecific pairwise distance against sequences from undescribed specimens in GenBank showed close similarities, except for the specimen identified as Versteria sp., which had 2–3 times the number of substitutions (Table 2). Interspecific pairwise distances between our samples and sequences from V. mustelae in Eurasia were 10 times that of the intraspecific distances (Table 2). Sequences from cox1 gene were also very similar to sequences previously identified as Versteria sp. from North America, with an average pairwise distance of 0.016 (cox1) (KF303340, KT223034, MK681866). Our samples also had an average interspecific pairwise distance of 0.025 compared against V. cuja (OL345572) and 0.104 compared against examples of V. mustelae from Eurasia (MW476516, NC021143) and a specimen of Versteria sp. from an ermine in Wisconsin that is related to the European clade (KT223035) (Lee et al. Reference Lee, Wallace, Clyde, Gendron-Fitzpatrick, Sibley, Stuchin, Lauck, O’Connor, Nakao, Lavikainen, Hoberg and Goldberg2016). This is similar to previous analysis by Bagnato et al. (Reference Bagnato, Gilardoni, Martin and Digiani2022), which calculated an intraspecific average distance of 0.011 among undescribed specimens in North America, compared to an interspecific difference of 0.024 with V. cuja and 0.093 with V. mustelae.
Table 2. Intra- and interspecific pairwise distance among Versteria in the nad1 gene. Sequences collected are identified in the left column, including their location (AB=Alberta, BC=British Columbia) and host species. All are adult specimens unless indicated as metacestodes, which were collected from the host liver. One sequence is identified as Versteria sp. due to high pairwise distance, which may imply it is a unique species. Sequences compared against all other sequences of V. rafei n. sp. collected in this study to provide an average intraspecific pairwise distance, then against sequences from GenBank. All GenBank ascension numbers are included. Analyses were conducted using the Tamura-Nei model (Tamura and Nei Reference Tamura and Nei1993). This analysis involved 13 nucleotide sequences. Codon positions included were 1st+2nd+3rd+Noncoding. All ambiguous positions were removed for each sequence pair (pairwise deletion option). There were a total of 451 positions in the final dataset. Evolutionary analyses were conducted in MEGA11 (Tamura et al. Reference Tamura, Stecher and Kumar2021)

The authors of the description of V. cuja noted that due to lower genetic distances between their specimen and other North American samples identified as Versteria sp., the possibility of conspecificity should not be ignored (Bagnato et al. Reference Bagnato, Gilardoni, Martin and Digiani2022). However, due to strong clustering and consistent mean pairwise distances between multiple samples from North America and the sequences from V. cuja in South America, and distinct morphological differences, mainly the presence of an armed cirrus in V. rafei n. sp., we are confident in asserting that they represent two distinct species in the American Clade.
Discussion
The species described herein belongs to the genus Versteria (Taeniidae), forming a clade with other specimens of Versteria in North America and V. cuja (Bagnato et al. Reference Bagnato, Gilardoni, Martin and Digiani2022). This clade included adult specimens identified as Versteria sp. in an ermine (M. erminea) in Colorado and a mink (N. vison) in Oregon (Lee et al. Reference Lee, Wallace, Clyde, Gendron-Fitzpatrick, Sibley, Stuchin, Lauck, O’Connor, Nakao, Lavikainen, Hoberg and Goldberg2016), and larval infections in muskrat (O. zibethicus) from Pennsylvania (Niedringhaus et al. Reference Niedringhaus, Ganoe, Lovallo, Walter, Yabsley and Brown2022), an orangutan (P. pygmaeus) at the Milwaukee County Zoo (Goldberg et al. Reference Goldberg, Gendron-Fitzpatrick, Deering, Wallace, Clyde, Lauck, Rosen, Bennett, Greiner and O’Connor2014), and humans in New Brunswick (Barkati et al. Reference Barkati, Gottstein, Müller, Sheitoyan-Pesant, Metrakos, Chen, Garceau, Libman, Ndao and Yansouni2019) and Pennsylvania (Lehman et al. Reference Lehman, Leal, Procop, O’Connell, Shaik, Nash, Nutman, Jones, Braunthal, Shah, Cruise, Mukhopadhyay and Banzon2019). According to the results of our molecular analysis, it is likely all these infections were attributable to Versteria rafei n. sp. and should be considered as such.
Human alveolar and cystic echinococcosis as well as taeniid cysticercosis and coenuruses have historically been diseases of concern for both humans and wildlife. These pathologies may be caused by a variety of cestode species (Deplazes et al. Reference Deplazes, Eichenberger and Grimm2019). Notoriously difficult to identify in their larval form, many cases are simply recorded as ‘echinococcosis’ or ‘cysticercosis’ without species-level identification. The genus Versteria was erected based on molecular characterisation of Taenia, which clustered with Versteria mustelae as a closer relative of Echinococcus than Taenia (Nakao et al. Reference Nakao, Lavikainen, Iwaki, Haukisalmi, Konyaev, Oku, Okamoto and Ito2013). Using molecular approaches, recent reports have identified diseases similar to alveolar and cystic echinococcosis caused by an unknown species of Versteria in humans as well as an orangutan in a zoo in North America, suggesting it should be considered as a differential diagnosis for cases of ‘echinococcosis’ (Barkati et al. Reference Barkati, Gottstein, Müller, Sheitoyan-Pesant, Metrakos, Chen, Garceau, Libman, Ndao and Yansouni2019; Deplazes et al. Reference Deplazes, Eichenberger and Grimm2019). In one such case, a patient even tested positive for two different ELISA tests for Echinococcus granulosus antigens, though negative for a confirmatory western blot (Barkati et al. Reference Barkati, Gottstein, Müller, Sheitoyan-Pesant, Metrakos, Chen, Garceau, Libman, Ndao and Yansouni2019), highlighting the potential for misidentification. However, this has only been recently discovered, and it is impossible to determine how many historical cases of ‘echinococcosis’ or ‘cysticercosis’ are due to Versteria, or how significant a threat it is to human communities.
Here, we document infections of adult Versteria rafei n. sp. in both river otter and mink from Alberta and British Columbia, as well as larval infections in the livers of mink and muskrat from Alberta. Our report is the first to document infection on a broad scale for river otter and mink, with infection intensities as high as 54 worms in a single individual host. The prevalence and intensity of infection as well as the presence of mature and gravid proglottids confirm both L. canadensis and N. vison as competent definitive hosts for the newly named species. Due to historical confusion over identification of Versteria species, it is hard to definitively identify the intermediate hosts of Versteria rafei n. sp. based on the literature, but previous reports of ‘T. mustelae’ and ‘T. tenuicolis’ identified multiple rodent species including beaver and muskrat in North America (Locker Reference Locker1955; Freeman Reference Freeman1956). We found larval cysts of taeniid parasites in the livers of muskrats, which were confirmed as Versteria rafei n. sp. based on molecular characterisation. Sequences and morphology matched those of metacestodes previously identified in muskrat by Niedringhaus et al. (Reference Niedringhaus, Ganoe, Lovallo, Walter, Yabsley and Brown2022), confirming O. zibethicus as an intermediate host. Muskrats are prey items for both river otter and mink, though mink more often include terrestrial rodents in their diet (Larivière and Walton Reference Larivière and Walton1998; Larivière Reference Larivière1999). Infection prevalence and intensity were slightly higher in mink, which may be explained by this dietary difference. Surprisingly, we also observed adult specimens of V. rafei in the intestines of two muskrat, and larval infections in mink. Taeniids are known to cause both adult and larval infections in the same species, such as with Taenia in humans and Echinococcus in dogs (Ito Reference Ito1997; Peregrine Reference Peregrine2015), and V. rafei may exhibit similar plasticity in its hosts. Muskrat, while predominantly feeding on vegetation, are also scavengers that have been reported to participate in cannibalism in winter when food is scarce (Errington et al. Reference Errington, Siglin and Clark1963). However, adult infection in muskrats is likely a rare occurrence.
The pathway of infection by metacestodes in mink is unclear. It should be noted that all mink observed with larval infections were also concurrently infected by adults of V. rafei. In one case, a mink from Alberta was infected with 21 adults and over 50 cysts in its liver. While we cannot definitively state the reason for this, it may indicate that hosts can be autoinfected by cysticerci; autoinfection via reverse peristalsis of gravid proglottids into the stomach or release of eggs in the intestine has been documented in other taeniid species. Versteria’s closest relatives, Echinococcus and Taenia, have been shown to autoinfect their hosts, in both canids and humans (Ito Reference Ito1997; Peregrine Reference Peregrine2015). If humans are competent definitive hosts for adults of V. rafei, this may be a threat for first nation groups in Canada who hunt and eat muskrats and may thus be infected with adults and potentially autoinfected by cysticerci (Wein and Freeman Reference Wein and Freeman1995; McLachlan Reference McLachlan2014). Infections may also be of concern for trappers handling wild mustelids, which could lead to contact with eggs and therefore infection by cysticerci.
Investigating the life history and prevalence of zoonotic pathogens in the wild is essential to prevent future infections. Infections of larval Versteria rafei n. sp. can be fatal in various hosts, including humans, captive animals, and wildlife (Lee et al. Reference Lee, Wallace, Clyde, Gendron-Fitzpatrick, Sibley, Stuchin, Lauck, O’Connor, Nakao, Lavikainen, Hoberg and Goldberg2016; Barkati et al. Reference Barkati, Gottstein, Müller, Sheitoyan-Pesant, Metrakos, Chen, Garceau, Libman, Ndao and Yansouni2019; Lehman et al. Reference Lehman, Leal, Procop, O’Connell, Shaik, Nash, Nutman, Jones, Braunthal, Shah, Cruise, Mukhopadhyay and Banzon2019; Niedringhaus et al. Reference Niedringhaus, Ganoe, Lovallo, Walter, Yabsley and Brown2022). Considering infection presents the same as Echinococcus species in humans (Barkati et al. Reference Barkati, Gottstein, Müller, Sheitoyan-Pesant, Metrakos, Chen, Garceau, Libman, Ndao and Yansouni2019), it is possible infections by Versteria rafei n. sp. are more prevalent than reported and currently diagnosed as echinococcosis. Versteria rafei n. sp. has also been reported to encyst in patients’ brains (Lehman et al. Reference Lehman, Leal, Procop, O’Connell, Shaik, Nash, Nutman, Jones, Braunthal, Shah, Cruise, Mukhopadhyay and Banzon2019). Since histology is not an accurate guide to the identification of larval cestode infections, molecular characterisation is essential to understand infection pathways and factors that may predispose people to infection by Versteria and other taeniid species. Such an approach is also needed to accurately identify the prevalence of specific parasites that may present with the same pathology but are transmitted by completely different organisms (Niedringhaus et al. Reference Niedringhaus, Ganoe, Lovallo, Walter, Yabsley and Brown2022).
Acknowledgements
We wish to thank Dr. Rafe Payne, aka ‘The Old Pirate’, for his almost 50 years of teaching and inspiring university students. Too often, we value only publication credits, forgetting the importance of teaching and the impact quality education has on our understanding of the natural world. Dr. Payne trained multiple generations of scientists, was a champion of scientific literacy in non-STEM majors, and more than anything else, loved his students. On a personal level, he inspired the first author of this paper to become a parasitologist and was a mentor in a time of need. Without Dr. Payne’s compassion and care for his students, the first author would not have pursued a career in research that led to the description of this new species. We give him this honour because he gave up his chance for scientific accolades to foster that opportunity for others. Unfortunately, Dr. Payne passed away October 19, 2023, while this manuscript was in review. He will be sorely missed.
We would also like to acknowledge and thank the Indigenous communities and Métis Nations, whose traditional lands provided the river otter, mink, and muskrat used for our study. Many thanks to Dr. Philippe Thomas at Environment and Climate Change Canada for his support both financially and logistically, Cait Nelson at the BC Wildlife Program for her hard work and support for this project, Melissa Todd, and the staff of the Coast Area Research Station and the Wildlife Health Program, BC Ministry of FLNRORD (Nanaimo), for their collaboration and support. Many thanks as well to the Alberta and British Columbia Trapper’s Associations for their logistical support in collecting animals and the individual trappers who contributed to this project. Thank you as well to Dr. Eric Hoberg and Dr. Tony Goldberg for their advice and assistance in the description process, especially Dr. Hoberg who took time to stain and mount some samples.
Author contribution
KMS designed the research, dissected hosts, identified the parasites, conducted the phylogenetic analysis, and wrote the manuscript. JB extracted and processed DNA samples. SG and CL funded and supervised the work. BP studied and identified the parasites, drew Figure 1, and advised as an expert taxonomist.
Financial support
This research was funded in part by BIOSCAN: Tracing the Patterns of Life on a Changing Planet via the Government of Canada New Frontiers in Research Fund (Transformation) and by Environment and Climate Change Canada.
Competing interest
None.
Ethical standard
None








