Introduction
Amphibians represent a very important group of vertebrates due to their presence in a broad range of aquatic and terrestrial habitats and their ecological roles as predators, prey, and hosts of a variety of organisms (Wells, Reference Wells2007; Pough et al., Reference Pough, Andrews, Crump, Savitzky, Wells and Brandley2016). These vertebrates are parasitized by different endo- and ectoparasitic helminths, and play roles as intermediate, definitive, and paratenic hosts in their life cycles (Koprivnikar et al., Reference Koprivnikar, Marcogliese, Rohr, Orlofske, Raffel and Johnson2012).
As a country, Mexico ranks fifth in richness of amphibians in the world with 376 species, and has a very high level of endemism (Parra-Olea et al., Reference Parra-Olea, Flores-Villela and Mendoza-Almeralla2014). However, the study of the helminth fauna of Mexican amphibians has followed a relatively low pace when compared to the study of helminth parasites of all other groups of vertebrates in the country, except birds (Pérez-Ponce de León et al., Reference Pérez-Ponce de León, García-Prieto, Mendoza-Garfias, Grillo and Venora2011). This situation is exacerbated by the global decline of amphibian populations, which is particularly acute in Mexico. A high percentage of species are threatened due to land use change (resulting in fragmentation, degradation, and habitat loss), plus emerging infectious diseases, introduced species, and over-exploitation (Frías-Álvarez et al., Reference Frías-Álvarez, Zúñiga-Vega and Flores-Villela2010).
The first known helminth species parasitizing an amphibian in Mexico (the nematode Hedruris siredonis infecting the Mexican axolotl, Ambystoma mexicanum), is coincidentally the first species of helminth recorded in the country, and was described by the English naturalist Baird (Reference Baird1858). Since then, the helminthological information generated for Mexican amphibians has been mostly represented by isolated taxonomic reports (e.g. Caballero, Reference Caballero1933; Lamothe-Argumedo, Reference Lamothe-Argumedo1973; Velarde-Aguilar et al., Reference Velarde-Aguilar, Romero-Mayén and León-Règagnon2014) and by description of the helminth fauna of a species of host in a particular location (e.g. Pulido-Flores, Reference Pulido-Flores1994; Trejo-Meléndez et al., Reference Trejo-Meléndez, Osorio-Sarabia, García-Prieto and Mata-López2019). Helminthological studies of a host species over its complete distributional range have never been pursued in Mexico, and only one work has explored helminth fauna of a species of host (the Sabinal frog Leptodactylus melanonotus) in a large number of localities in the country (see Mata-López et al., Reference Mata-López, León-Règagnon and García-Prieto2013).
To contribute to a better understanding of the helminth–amphibian association in Mexico, two studies have been carried out to compile the richness of this group of parasites. The first study by Pérez-Ponce de León et al. (Reference Pérez-Ponce de León, García-Prieto and Razo-Mendivil2002) analysed a database containing 460 records, represented by 119 species of helminths (including unidentified taxa), which were parasitizing 41 host species. The second one by Paredes-León et al. (Reference Paredes-León, García-Prieto, Guzmán-Cornejo, León-Règagnon and Pérez2008) presented the first list of helminth species found in Mexican amphibians with notes on their geographic distribution nationwide. From a database of 723 records, these authors reported the helminth fauna of 54 nominal amphibian species, composed of 96 nominal helminth species as well as another 11 species related to accidental infections.
In the present study, we review the literature and compile an updated database of records from studies that report at least one species of helminth parasitizing amphibians in Mexico, we evaluate the geographical representativeness of the regions from which helminths of Mexican amphibians have been studied so far, and we analyse the approaches followed by researchers in the study of these helminths. Our goals are to (1) provide an updated overview of the helminth richness and composition in Mexican amphibians, (2) determine if the study of this host–parasite association has had significant progress in recent years, (3) obtain the first spatial representation of the regions studied in the country, (4) evaluate sampling gaps and biases in the distribution of helminthological records relative to host species richness in biogeographic provinces, and (5) summarize the approaches emphasized by researchers studying helminths of Mexican amphibians.
Material and methods
We captured information in a database in Microsoft Access 2010 software by means of a retrospective bibliographical search containing information on helminths of Mexican amphibians generated from 1858 to May 2021. We gathered sources of information by consulting electronic databases such as CAB Abstracts, Biological Abstracts, Scopus, Web of Science and TESIUNAM using combinations of the terms ‘Helminth’, ‘Parasite’, ‘Infection’, ‘Amphibian’, ‘Platyhelminthes’, ‘Cestoda’, ‘Trematoda’, ‘Monogenea’, ‘Nematoda’, ‘Acanthocephala’, ‘Hirudinea’, ‘Pentastomida’, ‘salamander’, ‘frog’, ‘caecilian’, ‘new species’, and ‘Mexico’, both in English and in Spanish. We eliminated from our search works with no information on the topics and kept studies with one or more helminthological records from Mexican amphibians. In addition to data from literature (books, book chapters, scientific articles, theses, and dissertations) we consulted the following parasite collections: Colección Nacional de Helmintos (CNHE), Instituto de Biología, Universidad Nacional Autónoma de México, Mexico City, Mexico; Harold W. Manter Laboratory of Parasitology (HWML), and US National Parasite Collection (USNM), Smithsonian Institution, Washington, DC. We considered species in phyla Platyhelminthes, Acanthocephala and Nematoda, plus Hirudinea (in phylum Annelida) and Pentastomida (in phylum Arthropoda) as helminths, following Hugot et al. (Reference Hugot, Baujard and Morand2001), and excluded other annelids or arthropods. We followed the helminth species’ nomenclature and classification from Gibson et al. (Reference Gibson, Jones and Bray2002), Jones et al. (Reference Jones, Bray and Gibson2005) and Bray et al. (Reference Bray, Gibson and Jones2008) for Trematoda, WoRMS (2021) for Monogenea, Caira & Jensen (Reference Caira and Jensen2017) for Cestoda, Amin (Reference Amin2013) for Acanthocephala, Anderson et al. (Reference Anderson, Chabaud and Willmott2009) and Gibbons (Reference Gibbons2010) for Nematoda, Oceguera-Figueroa (Reference Oceguera-Figueroa, Rogers, Damborenea and Thorp2020) for Hirudinea and Lagunas-Calvo et al. (Reference Lagunas-Calvo, García-Prieto, Osorio-Sarabia, León-Règagnon and Oceguera-Figueroa2020) for Pentastomida. We followed Frost (Reference Frost2021) for scientific host names.
To map locations with helminthological records of Mexican amphibians, we used geographic coordinates provided in publications or we assigned coordinates in Google Earth (2021) if unprovided. We used ESRI ArcGIS Pro (2020) to map localities on the 14 biogeographic provinces from the regionalization of Mexico delimited by Morrone et al. (Reference Morrone, Escalante and Rodríguez-Tapia2017; fig. 1), and we provide information on the number of records per region (considering nominal species of helminths and species identified at the genus level). For all reported host species and for 371 amphibian species that occur in Mexico, we obtained range distribution maps as shapefiles from NatureServe (Reference NatureServe2010). We calculated host species richness and amphibian species richness for each biogeographic province by analysing spatial overlap between amphibian ranges and provinces. To incorporate helminths, we only considered nominal species and larval stages identified to genus if no adult records were reported for that genus. We then performed Spearman's correlation analyses between helminth richness and host richness by biogeographic province, and between helminth richness and total amphibian richness by province. We also calculated the discovery effort of helminths relative to host species richness and to amphibian richness following the methodology of Jorge & Poulin (Reference Jorge and Poulin2018). For each province, we weighted helminth richness and host richness by total richness to obtain relative richness values, and we subtracted the relative host richness from relative helminth richness. The difference represents the relative parasite discovery effort or rate of discovery of parasites (Jorge & Poulin, Reference Jorge and Poulin2018). Negative values indicate a low rate of discovery, values around zero indicate strong proportionality between province host richness and helminth species, and positive values indicate high rates of discovery of helminths. We evaluated helminth discovery effort relative to total amphibian richness per province in the same way.

Fig. 1. Biogeographic provinces of Mexico modified from Morrone et al. (Reference Morrone, Escalante and Rodríguez-Tapia2017).
Lastly, we classified the studies found in our literature search based on approaches followed by researchers and identified the fields of study most explored in Mexico.
Results
Helminths of Mexican amphibians
We found a total of 165 studies that included at least one record of helminth in at least one species of Mexican amphibian. Our database contains 868 records of adult helminths, or 1303 when considering taxa in larval stages. Helminths in adult stage are represented by 126 nominal species while helminths in larval stages are represented by 56 taxa (with 18 identified at the species level). Overall, these helminths have been found in 66 host species.
Regarding amphibian orders, there are more studies that include reports of helminths from frogs and toads (Anura) than those from salamanders and axolotls (Caudata) or caecilians (Gymnophiona): 136, 30 and two studies, respectively. Only one of the two species of Gymnophiona present in Mexico has been studied (Dermophis mexicanus), and it hosts two helminth species: the trematode Telorchis patonianus and the nematode Aplectana mexicana. The nematode appears to be a specialist to these caecilians since it has never been found in other hosts, while T. patonianus mainly parasitizes reptiles (Thatcher, Reference Thatcher1963). Members of Caudata, represented by 15 studied species, are parasitized by 20 helminth species found in adult stage and one species in larval stage, whereas the 48 species of Anura studied so far harbour 113 helminth species in adult stage and 14 species recorded in larval stages – that is, almost 90% of the helminth fauna recorded in all Mexican amphibians studied up to date. From the amphibians with helminthological records, the two most frequently studied species are the cane toad, Rhinella marina, and the Montezuma's frog, Lithobates montezumae. These two anurans account for the highest number of helminth species reported by different authors: cane toads are parasitized by 37 species of helminths and Montezuma's frogs by 26 species (supplementary tables S1 and S2).
Adult helminths of Mexican amphibians
Helminths parasitizing Mexican amphibians are frequently found in adult stage and are usually identified at the species level. These helminths have been found in 14 specific organs or tissues in the hosts, and amphibians act as definitive hosts in the life cycle of these 126 helminth species (sensu Chubb et al., Reference Chubb, Ball and Parker2010). Seven of these helminth species have rarely been reported to infect amphibian hosts (supplementary table S1).
The most represented group of adult helminths inhabiting Mexican amphibians is Nematoda with 58 nominal species, followed by Trematoda with 53; the richness of the remaining helminth groups varies between one and seven nominal species. A remarkable number of type helminth species has been described for Mexican amphibians (68), which represents more than 50% of the species recorded in the country.
The helminth fauna parasitizing amphibians in Mexico was poorly explored from 1858 to 1929, when its formal study started in the country. This was followed by a period of slow scientific exploration and some species descriptions from the 1930s to the 1990s, while in the last two decades the number of helminth species descriptions increased more than 100% relative to the previous 140 years. Thereby, the annual taxonomic description rate went from 0.30 species of helminths per year in the first 140 years of study, to 3.04 in the last 23 years (fig. 2).
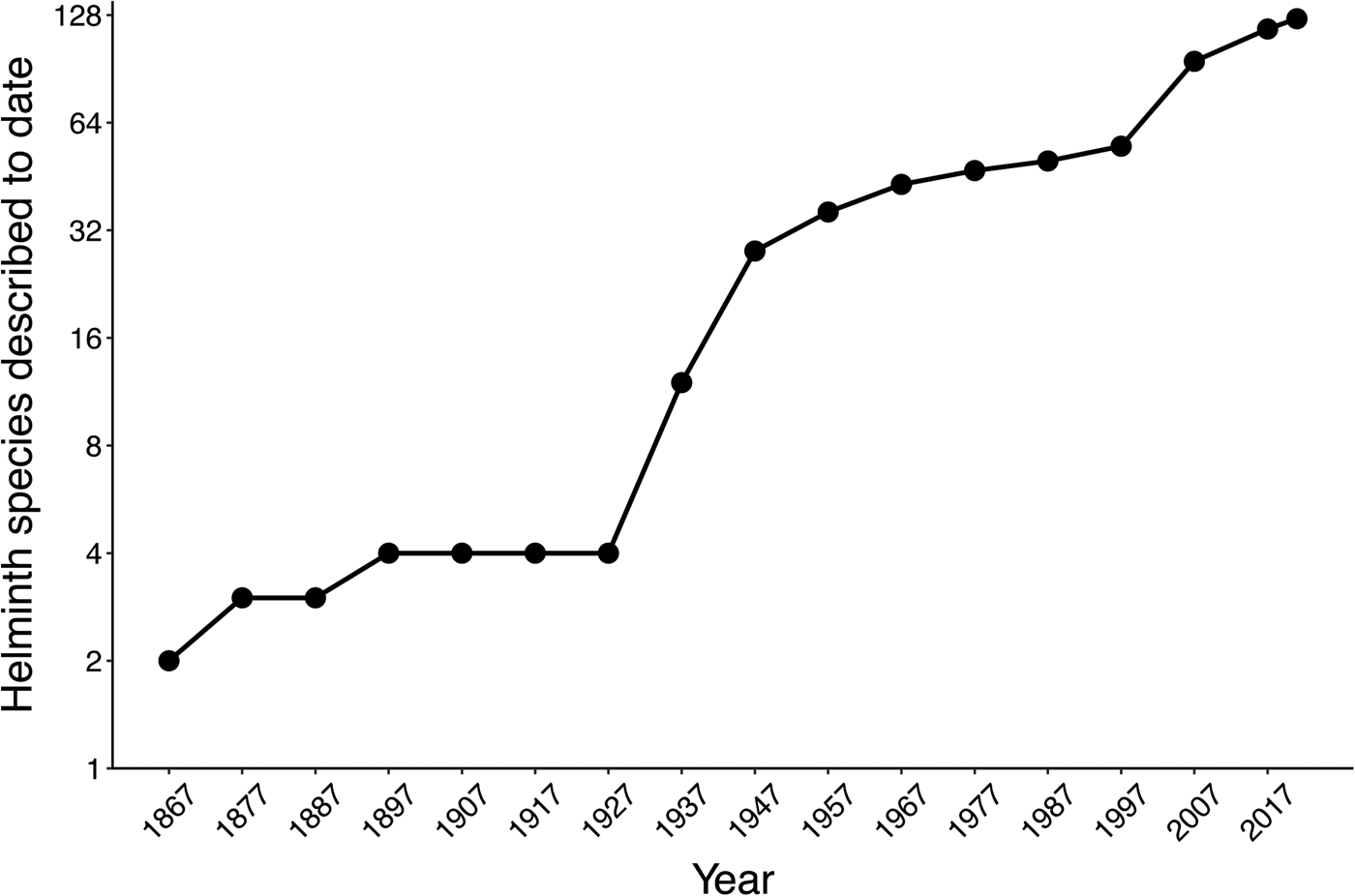
Fig. 2. Cumulative curve of helminth parasite species described for Mexican amphibians over time.
The helminth genera with the highest number of species in Mexican amphibians are Haematoloechus trematodes (19 species) and Rhabdias nematodes (ten species). These lung flukes and lung nematodes occur in 21 and 19 species of amphibians, respectively, and their hosts are mostly anurans (supplementary table S1). Other genera with lower richness such as Gorgoderina (eight species) and Aplectana (six species) infect a greater number of host species (22 and 24, respectively). In contrast, the six species of Ochoterenella distributed in Mexico seem specific to the bufonid R. marina, with a few sporadic records in hosts from the family Ranidae (supplementary table S1). The helminth species with the broadest host range is the gastrointestinal nematode Aplectana itzocanensis that parasitizes 16 species of anurans of five families, but it is not found in Caudata or Gymnophiona. The second broadest host range is that of the bladder fluke Gorgoderina attenuata that occurs in 15 host species: 11 anurans in four families and four salamanders in the genus Ambystoma, family Ambystomatidae. The gastrointestinal fluke Cephalogonimus americanus has the third broadest host range occurring in 13 host species: eight species of anurans in two families and five species of salamanders in the genus Ambystoma. On the other hand, almost 50% of helminth species have been found in a single host species. Determining whether this is due to host specificity or lack of sampling is an open line of investigation.
Larval helminths of Mexican amphibians
A total of 56 helminth taxa in larval stage have been found in 13 tissues/organs of Mexican amphibian hosts. These worms are commonly identified at the genus and even at the family taxonomic levels due to lack of diagnostic morphological characters expressed in the adult stage. Helminths in larval stages are more common in mesenteries and in the body cavity than adult helminths, and notably, suitable habitats in hosts, such as the heart and blood (where microfilariae of nematodes can be detected; McKenzie & Starks, Reference McKenzie and Starks2008) have been rarely examined (supplementary table S2).
The most represented group of larval helminths inhabiting Mexican amphibians is Nematoda with 17 genera, followed by Trematoda with ten genera and Acanthocephala with six genera. The most common larval helminths in these hosts are metacercariae of different trematodes, cystacanths in the genus Centrorhynchus (occurring in ten host species) and larval nematodes of the genera Contracaecum, Physaloptera, and Physocephalus (occurring in seven, nine, and nine host species, respectively; supplementary table S2).
The role of Mexican amphibians as hosts in the life cycles of larval helminths found so far includes paratenic (25 taxa), intermediate (19 taxa), definitive (four taxa that developed to adults in the host; sensu Chubb et al., Reference Chubb, Ball and Parker2010), and experimental (one species). These helminths use amphibians to reach the definitive host, where they mature and complete their life cycle. Seven taxa have rarely been reported parasitizing amphibian hosts (three of them identified at the family level), which makes it difficult to determine the role of the host in these helminths’ life cycle (supplementary table S2).
Geographic distribution of the helminthological records of Mexican amphibians
We retrieved geographic coordinates for 1090 helminthological records from Mexican amphibians out of 1303 total records (361 georeferenced by us). A variety of studies did not accurately specify study site and such records were not georeferenced.
The number of records per individual locality ranges from 1 to 54, and thus several points displayed on our map correspond to clusters of locality records. The map shows marked differences among biogeographic provinces, with clear distinction between the group of provinces with less than 40 records (ten provinces) and the Yucatán Peninsula province that contains 169 helminthological records. The provinces with more records are Veracruzan (289 records), Pacific Lowlands (229 records) and Transmexican Volcanic Belt (210 records), whereas the Californian, the Sonoran and the Sierra Madre Oriental provinces have no records or very low numbers of records: 0, 1 and 12, respectively (fig. 3).

Fig. 3. Localities with records of helminth species of Mexican amphibians on the 14 biogeographic provinces in the country. The colour scale corresponds to the number of helminthological records reported in each province, dark to light brownish provinces represent major to minor numbers of records.
Most of the helminthological records reported for the Veracruzan province correspond to the states of Veracruz and Oaxaca, whereas the majority of the records within the Pacific Lowlands correspond to the states of Guerrero and Jalisco, and those within the Transmexican Volcanic Belt are predominantly from the states of Mexico and Michoacán.
The highest number of nominal helminth species per province is 49, occurring in the Veracruzan province. Richness in this province is closely followed by that in the Pacific Lowlands province and the Transmexican Volcanic Belt province, both with 48 nominal helminth species parasitizing amphibians.
The helminth species that have been found in the most biogeographic provinces (seven) are the bladder fluke G. attenuata and the gastrointestinal nematode A. itzocanensis.
Gaps and biases in amphibian helminthological records with respect to host and amphibian species richness in biogeographic provinces
As indicated above, a few biogeographic provinces display a relatively high number of helminthological records from amphibians; however, this pattern does not necessarily reflect sampling gaps and biases related to host richness inhabiting a region (see Hopkins & Nunn, Reference Hopkins and Nunn2007; Jorge & Poulin, Reference Jorge and Poulin2018). We detected a statistically significant correlation between helminth richness and host richness by biogeographic province (r 2 = 0.62; P = 0.01) and between helminth richness and total amphibian richness by province as well (r 2 = 0.51; P = 0.05). The helminth discovery effort relative to host species richness had negative values in all provinces; we obtained the lowest discovery effort for Transmexican Volcanic Belt, Sierra Madre del Sur, and Balsas Basin provinces (−0.95, −0.83, and −0.82, respectively), and the highest values (yet still negative) for Yucatán and Baja Californian provinces (−0.02 and −0.06, respectively).
Regarding total amphibian richness per province, Sierra Madre del Sur had the highest value, followed by the Veracruzan and the Pacific Lowland provinces. The Baja Californian, the Californian and the Yucatan Peninsula provinces had the lowest amphibian richness in the country and thus, some of these provinces reached positive values for parasite discovery effort. Helminth discovery effort relative to amphibian species richness was negative in most provinces, being the lowest for Sierra Madre del Sur, Chiapas Highlands, and Balsas Basin provinces (−0.41, −0.22, and −0.16, respectively). Yucatan Peninsula, Pacific Lowlands and Baja Californian provinces had positive, but very low discovery effort values (0.15, 0.04, and 0.01, respectively; fig. 4).

Fig. 4. Discovery effort of helminths relative to amphibian species richness by biogeographic province in Mexico.
Research approaches in studies of helminths of Mexican amphibians
The vast majority of the 165 research works that included at least one helminthological record from a Mexican amphibian was performed with wild hosts in post metamorphic stages (164 studies). Only one work focused on experimental infections of tadpoles.
We identified 22 specific approaches followed by researchers. Most studies include only one approach (83.6%), some include two approaches (15.15%), and only two studies comprise three of these approaches (1.2%). We classified research approaches in three major fields of study: (1) Taxonomy; (2) Ecology and Pathology; and (3) Systematics and Phylogenetics (table 1).
Table 1. Research approaches followed in studies of helminth parasites of Mexican amphibians in order of frequency.
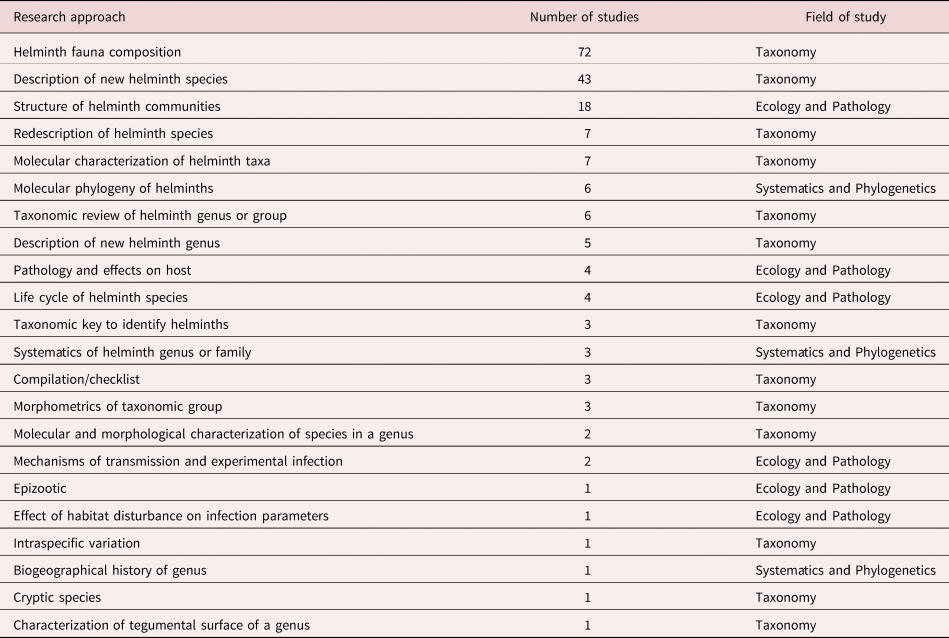
Differences among numbers of studies devoted to particular approaches are evident. Researchers have focused on describing the helminth fauna of a host species in a particular location, on the description of new species of helminths, and on the ecological analysis of helminth communities in hosts. Overall, studies following approaches in the taxonomic field are far more common than studies focused on ecology–pathology or on systematics–phylogenetics. Most studies have identified, described or classified helminth taxa from amphibians based on morphological characters, since incorporation of molecular techniques started in 1999 with a study by León-Règagnon et al. (Reference León-Règagnon, Brooks and Pérez-Ponce de León1999). Twelve works based on molecular data have been published so far (table 1).
Discussion
Helminth species of Mexican amphibians
Our updated database contains almost twice as many records as the numbers reported in Paredes-León et al. (Reference Paredes-León, García-Prieto, Guzmán-Cornejo, León-Règagnon and Pérez2008), which would seem to reflect a significant progress in our knowledge on helminth parasites of Mexican amphibians in recent years.
Seventeen species of trematodes, one species of acanthocephalan, 14 species of nematodes, and three species of pentastomids are records for amphibians produced in the last 13 years of research. However, the number of species of hosts examined has not changed much (54–66), reflecting that several recent helminthological works on amphibians have been conducted on host species previously studied. This common trend emphasizes the need to sample many more host species in order to capture reliable patterns of richness and diversity of helminth parasites of Mexican amphibians.
The majority of the helminthological records and helminth species reported in our review correspond to species of frogs and toads, which is expected considering that Anura is the richest order of amphibians in Mexico (Parra-Olea et al., Reference Parra-Olea, Flores-Villela and Mendoza-Almeralla2014). It is important to point out that elucidating the taxonomic identity of leopard frogs in the Rana pipiens complex inhabiting Mexico has been challenging, and thus, helminthological records associated to these frogs are not always assigned to described host species (e.g. Cabrera-Guzmán et al., Reference Cabrera-Guzmán, León-Règagnon and García-Prieto2007, Reference Cabrera-Guzmán, Garrido-Olvera and León-Règagnon2010). The groups or divisions of frogs comprising this complex are morphologically very similar and DNA sequencing has been necessary to better delimitate species (e.g. Ochoa-Vázquez et al., Reference Ochoa-Vázquez, Rosas-Valdez, Martínez-Salazar and Flores-Villela2019). Yet, more work is needed due to hybridization and presence of undescribed and cryptic species in the country (see Hillis, Reference Hillis1988; Zaldívar-Riverón et al., Reference Zaldívar-Riverón, León-Regagnon and Nieto-Montes de Oca2004).
The number of helminthological records in Caudata is relatively low in relation to the species richness of salamanders and axolotls in the country, which is high.
Helminths from Mexican axolotls, salamanders, frogs and toads that have been found in larval stages use amphibians mainly as intermediate hosts or as paratenic (transport) hosts (supplementary table S2) and need at least a subsequent host to complete their life cycle. These helminths have complex 2–5-host life cycles that usually start in a small short-lived invertebrate and involve trophic transmission, infecting successively larger intermediate hosts to then reach the definitive host, which is commonly an endothermic vertebrate (see Chubb et al., Reference Chubb, Ball and Parker2010; Benesh et al., Reference Benesh, Lafferty and Kuris2017, Reference Benesh, Parker and Chubb2021).
Larval nematodes often found in Mexican amphibians (supplementary table S2) usually start their life cycle in an aquatic or in a terrestrial arthropod (e.g. Contracaecum spp. and Physaloptera spp., respectively; Anderson, Reference Anderson2000). Common acanthocephalans such as Centrorhynchus sp. are also acquired by amphibian paratenic hosts via predation of arthropods (Benesh et al., Reference Benesh, Lafferty and Kuris2017). Interestingly, larval nematodes of the genus Gnathostoma, which includes species of importance for human health (Martínez-Cruz et al., Reference Martínez-Cruz, Bravo-Zamudio, Aranda-Patraca and Martínez-Marañón1989; García-Márquez et al., Reference García-Márquez, León-Règagnon, Lamothe-Argumedo, Osorio-Sarabia and García-Prieto2014) occur in frogs and toads (see supplementary table S2). However, and as expected, amphibians act mostly as definitive hosts for helminth species identified at species level. These helminths, found in adult stage, have direct or complex multi-host life cycles and infect amphibians trophically, through vectors (primarily mosquitoes), or by skin penetration (see Anderson, Reference Anderson2000; Langford & Janovy, Reference Langford and Janovy2009; Benesh et al., Reference Benesh, Lafferty and Kuris2017).
The comparatively high helminth richness reported for cane toads, R. marina, and Montezuma's frogs, L. montezumae, reflects biases related to high sampling effort. These two species of anurans are the two most studied species of Mexican amphibians from the helminthological point of view. Adult cane toads are large terrestrial anurans that breed in ponds, where tadpoles remain until metamorphosis. These toads are common and have a wide distributional range, being native to Latin America and introduced to other regions in the world. In Mexico, cane toads occur in eight biogeographic provinces along the Pacific and the Atlantic coasts and in the southern portion of the country. They are frequently found in disturbed areas, rural and suburban environments, and by river basins (Zug & Zug, Reference Zug and Zug1979; López et al., Reference López, Woolrich-Piña and Lemos-Espinal2009; Solís et al., Reference Solís, Ibáñez and Hammerson2009). Since individuals can be found in villages, are abundant, conspicuous and easy to collect, researchers commonly select cane toads to study their helminths (supplementary table S1).
Adult Montezuma's frogs are medium- to large-sized semi-aquatic anurans that breed in water bodies, where tadpoles develop. The species is endemic to Mexico and occurs in streams, temporary water bodies, and permanent lakes in the Sierra Madre Occidental province and in the Transmexican Volcanic belt province (Vázquez-Díaz & Quintero-Díaz, Reference Vázquez-Díaz and Quintero-Díaz2005; Ramírez-Bautista et al., Reference Ramírez-Bautista, Hernández-Salinas, García-Vázquez, Leyte-Manrique and Canseco-Márquez2009). Populations can be large in disturbed environments, and individuals are captured and consumed by people in some communities (Rodríguez-Blanco, Reference Rodríguez-Blanco1990; Quintero-Díaz et al., Reference Quintero-Díaz, Vázquez-Díaz, Sigala-Rodríguez, Ávilla-Villegas and Cruz-Aragón2008). Due to its distribution and cultural importance, researchers from different generations have selected these frogs to perform helminthological studies.
On the other hand, multiple reasons help explain the lack of helminthological surveys in a high number of amphibian species in Mexico. Nowadays, collecting and euthanizing amphibians to search for helminths in the country often requires permits issued by national/federal, regional, and local authorities. Examples of such authorities include Secretaría de Medio Ambiente y Recursos Naturales, the mayor of the municipality-location, and Comisariados de bienes comunales-ejidales in the villages, respectively. Importantly, some of the most charismatic and popular species of amphibians among Mexican herpetologists and parasitologists are under an IUCN (International Union for Conservation of Nature) threat category or are protected due to the conservation status of their populations or their habitats. If scientists aim to collect some of these species, applications need to be properly justified, but permits may not be granted or may allow collecting a low number of individuals in a particular location. Small sample sizes may not be adequate to represent and to report the helminth fauna of a host species of interest in a scientific publication, which is reflected in the remarkably high number of unstudied amphibian species. Since some Mexican communities represented by indigenous people have historically experienced arrival of non-locals who have extracted timber, soil, live plants or live animals from ecosystems (de Vos, Reference De Vos1988; Simonian, Reference Simonian1995; Wright & Leighton, Reference Wright and Leighton2002), in many instances local authorities only allow entrance of visitors practicing certified ecotourism with local guides to protect wildlife. In addition, some of the unstudied species of amphibians that inhabit pristine, or less disturbed or endangered vegetation types are restricted to remote areas with high elevation, complex topography and difficult access. Such areas are poorly explored, and herpetologists have found new species of secretive or minute amphibians, mostly salamanders (e.g. Hanken & Wake, Reference Hanken and Wake2001; Canseco-Márquez & Gutiérrez-Mayén, Reference Canseco-Márquez and Gutiérrez-Mayén2005; Parra-Olea et al., Reference Parra-Olea, Garcia-Castillo, Rovito, Maisano, Hanken and Wake2020) in these habitats, which increases the proportion of Mexican species for which helminth fauna is unknown.
Worldwide completeness is uncommon in host–helminth inventories (Poulin et al., Reference Poulin, Besson, Morin and Randhawa2016), and a distinctive feature of the helminth fauna associated with Mexican amphibians is the number of type species described. Finding new helminth species in different hosts has been common over the years, indicating that a high percentage of the helminths parasitizing this group of vertebrates is still unknown, and that more species will be found with more sampling. Thus, the information that we present in the supplementary checklists included in this paper is as a reflection of the current state of knowledge on the helminth–amphibian association in Mexico and it is expected to change.
We have identified the trematode genus Haematoloechus as having the highest number of species in Mexican amphibians. These flukes have a complex life cycle that usually involves a freshwater snail and an arthropod with aquatic life stages as first and second intermediate hosts, and then an amphibian as final host. Commonly, amphibians get infected by preying on adult dragonflies (Bolek et al., Reference Bolek, Detwiler, Stigge, Toledo and Fried2019). Most of the species recently identified and/or described from Mexican amphibians have been distinguished or authored by researchers from the group headed by Dr León-Règagnon using molecular characters in addition to morphology (e.g. León-Règagnon, Reference León-Règagnon2010; Velázquez-Urrieta et al., Reference Velázquez-Urrieta, Oceguera-Figueroa and León-Règagnon2019). This trend not only evidences the common occurrence and species richness of this genus in Mexican amphibians, but also reflects the importance of the scientific contributions of researchers specialized in taxonomic groups for the rate of species discovery and description.
The gastrointestinal nematode A. itzocanensis is the species of helminth that parasitizes the highest number of amphibian species in Mexico. All host species are anurans, including terrestrial, arboreal, semi-aquatic, and aquatic species. The life cycle of nematodes in this genus is direct and, thus, anurans get infected by ingesting larvae (Anderson, Reference Anderson2000). The bladder fluke, G. attenuata, exhibits the second broadest host range occurring in aquatic, semiaquatic, and terrestrial anurans and salamanders (supplementary table S1), which may be explained by its alternative life cycle strategies. Tadpoles can get infected by bladder flukes through direct ingestion of cercaria from clams, while post-metamorphic amphibians get infected by ingestion of metacercariae in damselfly second intermediate hosts, and by ingestion of infected anurans (Bolek et al., Reference Bolek, Snyder and Janovy2009). In addition, Velázquez-Urrieta & Pérez-Ponce de León (Reference Velázquez-Urrieta and Pérez-Ponce de León2021) indicated that specimens identified as G. attenuata in Mexico may comprise a cryptic species complex with similar morphology that require more molecular studies to be solved. Importantly, individuals of other species of Gorgoderina have been found in Mexican locations inhabited by G. attenuata, and individuals originally identified as G. attenuata have been recognized as undescribed species. Even though G. attenuata appears to be a species that parasitizes a broad range of hosts, more studies are necessary to clarify this common assumption (Velázquez-Urrieta & Pérez-Ponce de León, Reference Velázquez-Urrieta and Pérez-Ponce de León2021). The helminth with the third broadest hosts spectrum – the gastrointestinal fluke C. americanus – has also been found in several species of anurans and salamanders (supplementary table S1). Species of Cephalogonimus use snails as first intermediate hosts, and then cercariae penetrate aquatic larval amphibians as second intermediate hosts. Adult amphibians acquire the parasite by ingesting infected tadpoles or larval salamanders (Lang, Reference Lang1968; Dronen & Lang, Reference Dronen and Lang1974).
Parasites with broad ecological and phylogenetic host range and low specificity (sensu Lymbery, Reference Lymbery1989; Poulin & Mouillot, Reference Poulin and Mouillot2003), as the ones just described, are generalists – that is, they can exhibit tolerance to different physiological and behavioural characteristics of distantly related host species (Euzet & Combes, Reference Euzet and Combes1980). The helminth fauna of an important number of Mexican amphibians includes at least one generalist species that occurs in multiple hosts (supplementary table S1). Interestingly, some helminth genera typically found in amphibians in other Nearctic and Neotropical regions, such as members of Acanthocephalus and Schrankiana (Magalhães-Campião et al., Reference Magalhães-Campião, Honorio-Morais, Tavares-Dias, Aguiar, de Melo- Toledo, Roland-Tavares and da Silva2014), as well as the species Ribeiroia ondatrae (Roberts & Dickinson, Reference Roberts and Dickinson2012), have not been found in Mexican amphibians. These helminths may not have colonized temperate or tropical regions in Mexico due to geographical distance, unfavourable conditions, lack of hosts, low vagility of hosts, niche conservatism or constraints related to immune responses exhibited by hosts (see Wiens & Graham, Reference Wiens and Graham2005; Stephens et al., Reference Stephens, Altizer and Smith2016). Alternatively, they may have not been detected due to lack of sampling.
Geographic distribution of the helminthological records of Mexican amphibians
Some of the first researchers who studied helminth parasites of Mexican amphibians did not provide geographic coordinates or specific details on locations, but most works contain this information. A clear asymmetry in number of helminthological records is present among the biogeographic provinces of Mexico, with the Veracruzan, the Pacific Lowlands, and the Transmexican Volcanic Belt provinces having the highest number of helminthological records for amphibians.
The high number of records in the Veracruzan province can be mostly attributed to presence of Los Tuxtlas Tropical Biology Station, from Universidad Nacional Autónoma de México, in Los Tuxtlas Biosphere Reserve. Many researchers have conducted studies on helminth parasites of amphibians at Los Tuxtlas (e.g. Guillén-Hernández et al., Reference Guillén-Hernández, Salgado-Maldonado and Lamothe-Argumedo2000; Goldberg et al., Reference Goldberg, Bursey, Salgado-Maldonado, Báez-Valé and Cañeda-Guzmán2002; Paredes-Calderón et al., Reference Paredes-Calderón, León-Règagnon and García-Prieto2004), and hosts have been collected from tropical rainforest, cattle pastures, villages, and lagoons. This area alone accounts for 14.5% of all the georeferenced helminthological records from amphibians in the country.
The Pacific Lowlands province includes more than 60% of the country's coastline and has many locations and ports with large populations and important economic activities (Chiappa-Carrara et al., Reference Chiappa-Carrara, Enríquez, Papiol, Mariño-Tapia, Reyes-Hernández and Sheppard2018). A significant number of localities with helminthological records in this province are concentrated around villages and coastal lagoons in Acapulco de Juárez municipality (e.g. Cabrera-Guzmán et al., Reference Cabrera-Guzmán, León-Règagnon and García-Prieto2007) and in the tropical dry forest of Chamela Biological Research Station, Universidad Nacional Autónoma de México, Chamela-Cuixmala Biosphere Reserve (e.g. Galicia-Guerrero et al., Reference Galicia-Guerrero, Bursey, Goldberg and Salgado-Maldonado2000).
On the other hand, the high number of records in the Transmexican Volcanic Belt province – overlapping the center of the country west to east – is mostly related to studies performed by the early Mexican helminthologists who frequently worked around lakes of commercial and cultural importance, and adjacent areas in central Mexico. Examples of these locations are Ciénega de Lerma, state of Mexico (Caballero, Reference Caballero1942a, Reference Caballero1942b, Reference Caballero1942c), Lago de Xochimilco (Bravo-Hollis, Reference Bravo-Hollis1941; Caballero, Reference Caballero1947) and Contreras in Mexico City (Bravo-Hollis & Caballero, Reference Bravo-Hollis and Caballero1940; Bravo-Hollis, Reference Bravo-Hollis1943), among others.
Two helminth species – the fluke G. attenuata and the nematode A. itzocanensis – have been found in amphibians from seven Mexican biogeographic provinces each, and both species inhabit a high number of sympatric and allopatric host species as well (see above and supplementary table S1). Such generalism evidences efficient dispersal and tolerance to a broad spectrum of environmental conditions in a variety of habitats (A. itzocanensis), flexibility in the use of different intermediate hosts in the life cycle (G. attenuata) and likely large diet breadth of their amphibian hosts (see Park, Reference Park2019).
The helminth richness detected throughout Mexican biogeographic provinces is, to some extent, an artifact of sampling effort. Provinces that have been more frequently studied account for the highest numbers of nominal helminth species (Veracruzan province, Pacific Lowlands province and Transmexican Volcanic Belt province). Nevertheless, even these relatively well-sampled provinces require much more work and exploration, since records are concentrated in particular locations (fig. 3), and these provinces have high amphibian richness.
Gaps and biases in amphibian helminthological records with respect to host and amphibian species richness in biogeographic provinces
The significant positive correlations between helminth richness and host richness and between helminth richness and total amphibian richness support the expectation that sampling biogeographic provinces with higher amphibian richness would increase the likelihood of new records of helminths.
Our spatial analyses showed that most biogeographic provinces are strongly under-sampled, as measured by the helminth discovery effort. All provinces had negative discovery effort values relative to host richness, with three provinces (Transmexican Volcanic Belt, Sierra Madre del Sur, and Balsas Basin) having the lowest values. Sierra Madre del Sur, Chiapas Highlands and Balsas Basin provinces had the lowest discovery efforts relative to total amphibian richness. Thus, more helminth species are expected to be reported from Sierra Madre del Sur and Balsas Basin provinces in future research.
The slightly higher discovery effort values for Yucatan Peninsula and for Baja Californian provinces relative to host richness and total amphibian richness suggest that these two provinces are better sampled for helminth richness and perhaps should be given lower priority in future sampling efforts. It is important to point out that both provinces have relatively low amphibian richness.
Producing robust databases to describe spatial patterns of helminth diversity requires sampling of unexplored regions and host species in the Nearctic and the Neotropical areas of Mexico, and the results presented in this work (figs 3 and 4) aim to serve as tools that help visualize the existing gaps and to propose studies and directions to increase geographic representativeness. Most regions with low helminth discovery effort have high biological diversity, high amphibian richness or high levels of endemism for amphibians (Ochoa-Ochoa et al., Reference Ochoa-Ochoa, Campbell and Flores-Villela2014; present work); therefore, these regions potentially hold high helminthological diversity and undescribed species.
It is important to consider, however, that some unexplored regions in Mexico such as the Californian, the Sonoran, and the Tamaulipas provinces face important socioeconomic problems and violence related to drug cartels and the black market for gasoline/petrol. Thus, safety concerns may limit sampling particular areas and lead to more exploration along roads, highways or safer regions in the country (see Rodríguez-Mega, Reference Rodríguez-Mega2019).
Research approaches in studies of helminths of Mexican amphibians
More studies on helminth parasites of Mexican amphibians are necessary and we have detected clear gaps in the approaches followed by researchers who have studied these organisms. We also noted that some research works remain as unpublished theses or dissertations, documents that are often difficult to access, leading to loss of valuable information.
The lack of information on different aspects of the helminth–amphibian association is mostly related to the high number of undescribed species found by researchers when conducting helminthological studies. This situation leads to works and publications focused on the description of new species and slows down the exploration of other aspects and research avenues on these interactions. This is clearly evidenced by the number of new species of helminths described for the amphibian fauna of the country (68 of the 127 recorded in 163 years of studies). Since rate and efficiency of taxonomic description efforts are very important to characterize the helminth diversity in vertebrates (Carlson et al., Reference Carlson, Dallas, Alexander, Phelan and Phillips2020), helminth species descriptions are fundamental, but need to be complemented with the study of ecological, pathological and phylogenetic aspects of these parasites.
Worldwide, most of the research on amphibian pathogens has focused on chytrid fungus and on ranaviruses that have been recognized as factors associated with amphibian declines, while the role of helminth parasites in such declines has been poorly explored (Bienentreu & Lesbarrères, Reference Bienentreu and Lesbarrères2020). Regarding pathogenic helminths, research elsewhere has mostly been performed on nematodes of the genus Rhabdias and trematodes of the genera Ribeiroia and Echinostoma, which usually affect performance and/or development of amphibians (Koprivnikar et al., Reference Koprivnikar, Marcogliese, Rohr, Orlofske, Raffel and Johnson2012). Ten species of Rhabdias are present in Mexico (supplementary table S1), but no study has focused on their non-lethal or lethal effects on their hosts.
Three of the four investigations specifically related to pathogenic helminths parasitizing Mexican amphibians are descriptive studies of the lesions produced by larval nematodes of the genus Eustrongylides to Lithobates megapoda (Ramírez-Lezama & Osorio-Sarabia, Reference Ramírez-Lezama and Osorio-Sarabia2002) and A. mexicanum (Recuero et al., Reference Recuero, Cruzado-Cortes, Parra-Olea and Zamudio2010), and the genus Gnathostoma to Lithobates forreri (García-Márquez et al., Reference García-Márquez, León-Règagnon, Lamothe-Argumedo, Osorio-Sarabia and García-Prieto2014). The fourth study refers to death of an Ambystoma taylori due to damage and congestion of the digestive tract produced by H. siredonis nematodes (Michels et al., Reference Michels, Hernández-Díaz, Carmona-Muciño, Muñoz-García, Osorio-Sarabia, Acebes, Couchman, Owen and Waterman2016).
We did not find any long-term studies on amphibian helminthiasis or experimental studies in mesocosms in our review, and we found only one study that carried out experimental infections in the laboratory. In that study, tadpoles of the spadefoot toad Spea multiplicata were exposed to cercariae of the trematode Centrocestus formosanus to obtain mature metacercariae (Amaya-Huerta, Reference Amaya-Huerta1995).
Most studies in Mexico have described composition of helminth fauna in one or several host species and are based on natural infections occurring in wild hosts. Performing experimental studies and/or infections in the lab or in mesocosms often needs regulation by animal ethics committees, and this requirement may help explain the scarcity of experimental studies detected.
Overall, studies performed by helminthologists working with parasites of Mexican amphibians have focused on 10 main research approaches, mostly related to taxonomy (table 1). Gaining a better understanding of amphibian–helminth interaction in Mexico would require the exploration of various relevant fields in the near future. We suggest increasing the extent and scope of studies with the inclusion of the following approaches: (1) life cycles and/or mechanisms of transmission of helminths including characterization of free-living stages, if present; (2) lifecycle plasticity; (3) host–helminth phenological synchrony; (4) morphological and molecular characterization of larval stages; (5) effects of helminths on host growth, development, performance, behaviour and survival; (6) effect of host body size, sex, and age on parasitic loads; (7) effect of host population density on rates of parasitism; (8) helminth parasites of host larval stages and fate after metamorphosis; (9) diagnosis and pathogenic effects of infections (at cell, tissue, and organ-system levels) in captive and wild hosts; (10) immune responses to helminth infections; (11) patterns of seasonal prevalence of helminth species; (12) geographic variation in helminth community structure at different levels; (13) effect of abiotic and biotic factors on helminth community structure; (14) actual and potential geographic distribution of helminth species; (15) effects of anthropogenic disturbance on rates of parasitism; (16) response of amphibian–helminth interaction to climate change; (17) host specificity at phylogenetic and geographic scales, and host–helminth coevolution; (18) host shift; (19) biogeographical affinities of helminths and hosts; and (20) determination of cryptic species. The inclusion of such approaches in future studies may require funding and collaboration of researchers with different expertise.
To date, the most important initiative approved by authorities to study helminth parasites of Mexican amphibians in different regions was the project ‘The amphibians and reptiles and their parasites of Mexico, a megadiverse country’, funded by the US National Science Foundation (grant numbers DEB-0613802 and DEB-0102383 to Dr Jonathan A. Campbell), and carried out between 2001 and 2012. A high number of US and Mexican researchers and students from the University of Texas at Arlington and the Universidad Nacional Autónoma de México were involved. These researchers collected an important number of helminths from species of amphibians for which parasitic fauna was little known or entirely unknown. Even though 23 research articles have been published on helminths of amphibians collected in this project (which include the recent description of 13 new species; fig. 2), the largest proportion of these helminths remains unstudied. Most of these helminth specimens are housed in the CNHE, Mexico City.
On the other hand, collecting and euthanizing individuals of invasive species may be facilitated in some instances; thus, focusing on the study of helminth parasites of invasive species of amphibians offers another poorly explored research avenue. Species of amphibians that have been introduced to Mexico or to regions in Mexico can potentially introduce helminths with them. The American bullfrog Lithobates catebeianus, for example, has been introduced for aquaculture into different states in Mexico. Farming this species is common in the country, but individuals have the ability to escape from facilities and colonize new areas (Casas-Andreu et al., Reference Casas-Andreu, Aguilar-Miguel and Cruz-Aviña2001; Becerra-López et al., Reference Becerra-López, Esparza-Estrada, Romero-Méndez, Sigala-Rodríguez, Mayer-Goyenechea and Castillo-Cerón2017). There is no published information on the helminths parasitizing this species in invaded regions within Mexico, and we do not know if old and recently introduced populations have transmitted helminths to native species of amphibians.
It is important to highlight that officially registering helminth collections and making data from these collections widely and freely available to scientists in the country will help to reach common goals in the study of helminth parasites of Mexican amphibians (Pérez-Ponce de León et al., Reference Pérez-Ponce de León, García-Prieto and Razo-Mendivil2002). Some of the collections and research groups are currently working on this task, attempting to make databases accessible to researchers.
Guidelines for performing more comprehensive research on helminth parasites of amphibians
The findings and trends reported here for Mexico provide lessons that can help research groups performing helminthological studies in other parts of the world, particularly in the Neotropics. Most countries in Central and South America have limited knowledge of helminth parasites of amphibians and lack studies encompassing many of the approaches listed above. Costa Rica and Brazil have the most sampled amphibians from Central and South America, respectively. Costa Rica is a small diverse country, and 39% of its 207 amphibian species have at least one helminthological record (Rodríguez-Ortiz et al., Reference Rodríguez-Ortiz, García-Prieto and Pérez-Ponce de León2004; Bursey & Brooks, Reference Bursey and Brooks2010; Goldberg & Bursey, Reference Goldberg and Bursey2010). In Brazil, the largest South American country, less than 10% of the 946 amphibian species reported by 2014 had been sampled for helminths (see Magalhães-Campião et al., Reference Magalhães-Campião, Honorio-Morais, Tavares-Dias, Aguiar, de Melo- Toledo, Roland-Tavares and da Silva2014). In addition, more amphibian species have been found or described recently, so the current Brazilian amphibian richness comprises 1188 species (Segalla et al., Reference Segalla, Berneck and Canedo2021). In both countries, most of the studies have focused on descriptive taxonomic approaches, like in Mexico.
Planning and modifying the way to initiate and conduct projects is crucial to improve research on amphibian helminths worldwide, to increase knowledge, and to accomplish broader impacts. This would be particularly important in countries that have mostly or exclusively worked in taxonomy.
Based on our review, it is pertinent to recommend researchers interested in studying helminth parasites to consider the following steps: (1) review published literature in the country to identify priority research needs; (2) select study system, including host species and location(s); (3) set goals and/or form hypotheses connected to at least three research approaches; (4) determine appropriate sample sizes, sampling effort, and duration of the study; (5) incorporate innovative perspectives and contact potential collaborators with different taxonomic expertise and research focus; (6) estimate costs and apply for funding; (7) train students during field and laboratory work; (8) report quantitative results and include statistical analyses, as opposed to only descriptive information; (9) write one or more scientific manuscripts, preferably in English; and (10) select scientific journals that are accessible to readers in different countries to submit manuscript(s) for publication.
We acknowledge that finding funding agencies and funding opportunities is challenging in many countries in the Americas. However, projects may not need a large budget if collaborations are established with universities or institutions that already have equipment, materials, and supplies for particular research needs.
Importantly, researchers must be prepared to find helminth parasites that are undescribed species. In this case, they should recognize that the more scientists involved in the description of a species, the higher its quality typically is. Thus, we advise collaboration among experts (see also Poulin & Presswell, Reference Poulin and Presswell2016), such that morphological and molecular taxonomic work may proceed alongside research on ecological, etiological or evolutionary fields. Researchers will be able to devote efforts to more approaches and to maximize the amount of information obtained from helminths, tissues and any samples collected from hosts following our proposed strategy.
In conclusion, we have identified gaps and biases in the study of helminth parasites of Mexican amphibians, which highlights the need for further research. The high richness of amphibians and helminths in Mexico offers opportunities to investigate challenging biological systems threatened by anthropogenic disturbance, and we hope that our research can encourage Mexican students and professors to start studies focused on the gaps we identified and to extend research projects focused on helminths of amphibians. Investigating and describing helminths of amphibian species that have not been studied, exploring regions that lack studies and considering different perspectives, techniques and approaches are essential to increase our understanding of the ecological and evolutionary importance of the helminth–amphibian association within ecosystems.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S0022149X21000614
Acknowledgements
We thank Daniel Moen for advice and Georgina Ortega-Leite for providing bibliographic references. Two anonymous reviewers for providing helpful comments.
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflicts of interest
None.
Ethical standards
Considering the bibliographic nature of this review, the authors assert that are referencing all citations of the published works related to the study. No animals were collected, no animals were kept in captivity, and no experiments were performed for this research.







