Introduction
Livestock farming is critical to the socioeconomic development of sub-Saharan Africa because it provides food security, income production, and livelihood support to millions of people, particularly in resource-poor livestock farming communities (Erdaw, Reference Erdaw2023). Parasitic infections, on the other hand, pose a severe threat to cattle health and productivity, resulting in huge economic losses for the region (Phiri et al., Reference Phiri, Chota and Phiri2007, Zvinorova et al., Reference Zvinorova, Halimani, Muchadeyi, Matika, Riggio and Dzama2016; Chongmobmi & Panda, Reference Chongmobmi and Panda2018). Amphistomes commonly known as “conical flukes” have emerged as a major source of concern globally, affecting a variety of domesticated ruminant species such as cattle, sheep, and goats (Horak, Reference Horak1971; Pfukenyi & Mukaratirwa, Reference Pfukenyi and Mukaratirwa2018). Hence, they have been recognised for their major threats to sub-Saharan Africa’s livestock industry, causing significant economic losses due to stunted growth including death in young animals, low food conversion rates, poor milk, meat, and wool production, and poor hide and skin quality (Pfukenyi et al., Reference Pfukenyi, Mukaratirwa, Willingham and Monrad2005; Bunza et al., Reference Bunza, Ahmad and Fana2008). Despite these negative impacts, amphistomosis is still a neglected tropical disease of ruminant livestock (Hotessa & Kanko, Reference Hotessa and Kanko2020).
Amphistomes are digenetic trematodes from the subfamily Paramphistomoidea Fischoeder, 1901 (Lotfy et al., Reference Lotfy, Brant, Ashmawy, Devkota, Mkoji and Loker2010; Mitchell et al., Reference Mitchell, Zadoks and Skuce2021). They have a heteroxenous life cycle, with aquatic snails serving as obligatory intermediate hosts and ruminants as definitive hosts. More than 70 amphistome species have been recorded globally (Ghatani et al., Reference Ghatani, Shylla, Tandon, Chatterjee and Roy2012) and in sub-Saharan Africa, 36 species have been documented in wild ruminants (Sibula et al., Reference Sibula, Nyagura, Malatji and Mukaratirwa2024); the majority of these species is shared between both domestic and wild ruminants (Pfukenyi & Mukaratirwa, Reference Pfukenyi and Mukaratirwa2018; Sibula et al., Reference Sibula, Nyagura, Malatji and Mukaratirwa2024). Adult amphistomes inhabit the digestive system of ruminants specifically in the rumen and reticulum (Sibula et al., Reference Sibula, Nyagura, Malatji and Mukaratirwa2024), and immature stages cause intestinal amphistomosis, also known as amphistomiasis (Pfukenyi & Mukaratirwa, Reference Pfukenyi and Mukaratirwa2018). Adult amphistomes cause localised loss of rumen papillae, whereas immature flukes penetrate the mucosa of the duodenum and upper ileum to plug feed, causing necrosis and haemorrhagic duodenitis, resulting in severe pathological changes (Mavenyengwa et al., Reference Mavenyengwa, Mukaratirwa, Obwolo and Monrad2005; Opara et al., Reference Opara, Chikezie, Udoidung, Yaro, Onwumerobi and Afia2017). The immature amphistomes may cause significant mortality rates in domesticated ruminants, reaching up to 80% to 90% (O’Shaughnessy et al., Reference O’Shaughnessy, Garcia-Campos, McAloon, Fagan, de Waal, McElroy, Casey, Good, Mulcahy, Fagan and Murphy2018; Pfukenyi & Mukaratirwa, Reference Pfukenyi and Mukaratirwa2018).
This disease has a global distribution; however, the prevalence and intensity of infection differ by country and location (Ghatani et al., Reference Ghatani, Shylla, Tandon, Chatterjee and Roy2012; Pfukenyi & Mukaratirwa, Reference Pfukenyi and Mukaratirwa2018; Nyagura et al., Reference Nyagura, Malatji and Mukaratirwa2024). Climate and local environmental variables, such as humidity, temperature, rainfall, and vegetation, have an impact on the prevalence and intensity of infection in pasture (Hajipour et al., Reference Hajipour, Mirshekar, Hajibemani and Ghorani2021; Sibula et al., Reference Sibula, Nyagura, Malatji and Mukaratirwa2024). The epidemiological pattern of amphistome infection in domestic animals is impacted by production management systems, grazing behaviour of animals, and the presence of infected definitive hosts (Horak, Reference Horak1971) and the intermediate host species (Sibula et al., Reference Sibula, Nyagura, Malatji and Mukaratirwa2024). The prevalence of infection may differ based on the age, sex, and physiological state of an animal, animal species, and between seasons (Horak, Reference Horak1971; Kanyari et al., Reference Kanyari, Kagira and Mhoma2010; Pfukenyi & Mukaratirwa, Reference Pfukenyi and Mukaratirwa2018).
Although several field-based studies have been conducted to determine the prevalence of amphistome infections in livestock in sub-Saharan Africa, the prevalence estimate for the region is not known. A complete synthesis of current data on amphistome prevalence in domestic ruminants across sub-Saharan Africa through a systematic review and meta-analysis is critical as it can offer a more accurate estimation of the disease burden, identify high-risk locations, and assist in the design of evidence-based interventions for prevention and control.
Materials and methods
Search strategy
Four electronic databases, Google Scholar, PubMed, Science Direct, and Web of Science, were used to conduct a systematic literature search for peer-reviewed publications from 2002 to 2023, following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. Boolean operators (AND, OR) were used in conjunction with the following selected key terms; amphistome infection OR amphistomosis OR paramphistome infections OR paramphistomosis, rumen flukes OR conical flukes AND domestic ruminants OR livestock OR cattle OR sheep OR goats, AND prevalence in sub-Saharan Africa. Related studies were retrieved based on a preliminary screening of the titles and abstracts. Additional studies were searched manually through cross-referencing of the eligible studies. The literature search was limited to English. All full-text articles selected were imported and managed in the EndNote reference manager version X8 (Clarivate Analytics, Philadelphia, PA, USA).
Inclusion and exclusion criteria
The inclusion criteria were: (1) the study was carried out in sub-Saharan Africa, (2) the host animal (domestic ruminant) was indicated, (3) the sample size and number of positive cases clearly stated, (4) the prevalence was based on natural infections, and (5) diagnostic/identification method was clearly stated.
Excluded from this review were articles that reported on amphistome infections in non-ruminants, amphistome infections in wildlife, and studies conducted outside sub-Saharan Africa. Furthermore, articles published in other languages besides English, or outside the period of study, duplicate studies, studies without full-texts, reviews, unpublished reports, conference abstracts, and dissertations were also excluded.
Data extraction
To eliminate bias in the literature search, the retrieved studies were thoroughly checked independently by two reviewers (I.N. and M.P.M.). Thereafter, articles not fulfilling the inclusion criteria, duplicate, and low-quality assessed articles were excluded from meta-analysis. For meta-analysis, data extracted from text, tables, and figures were computed and processed in MS Excel with the authors’ names, published year, location, animal species, diagnostic/identification method, the number of animals examined, case positives, and prevalence rate.
Quality assessment
The quality of each study for meta-analysis was evaluated independently following the Grading of Recommendations Assessment, Development, and Evaluation methods criteria by Guyatt et al. (Reference Guyatt, Oxman, Vist, Kunz, Falck-Ytter, Alonso-Coello and Schünemann2008). Studies that met the inclusion criteria outlined were assigned a score of 1 point if complete information was provided and 1 point for each subsequent inclusion criterion that was met. Thus, all the studies were given a score between 0 and 5 points. Publications with a total score of 5 points were deemed good quality, 4 moderate quality, and ≤3 as low quality and were excluded. The standardized quality index score (between 0 and 1) based on quality was then computed (supplementary table 1).
Meta-analysis and meta-regression
Prevalence data were transformed using the double arcsine method to avoid overestimating the weight of individual studies (Barendregt et al., Reference Barendregt, Doi, Lee, Norman and Vos2013). The MetaXL add-in for Microsoft Excel (www.epigear.com) was used to assess the quality effects model to account for the heterogeneity. Heterogeneity between estimates was evaluated using the inverse variance statistic (I 2 index), and its significance was tested using Cochrane’s Q test. Following Higgins et al. (Reference Higgins, Thompson, Deeks and Altman2003) protocol, the I 2 score of 25%, 50%, or 75% was interpreted as low, moderate, or high heterogeneity, respectively. Forest plots were used to graphically demonstrate the estimated prevalence and the 95% confidence interval (CI) of amphistome among hosts. To evaluate the prevalence estimates for the major subgroups, meta-analysis was computed for geography (region), animal host, detection method, age (young and adult), sex (male and female), body condition score (BCS), seasonality (wet and dry), and years. Funnel plots were used to evaluate the publication bias. IBM SPSS Statistics 28.0 was used for all subsequent statistical analyses. To identify the sources of heterogeneity, univariate meta-regression was performed with region, host, sex, age, body condition factor, study type and study period fixed as independent factors. The meta regression was treated as linear model on the logit transformed prevalence data. The linear regression analysis was conducted to evaluate publication bias using Egger’s test. The Trim and Fill approach was employed after the Egger’s test to evaluate the possible influence of publication bias on the total effect size.
Results
Search results and characteristics of eligible studies
A literature search on Google Scholar, PubMed, Science Direct, and Web of Science generated 1571 records (Fig. 1). Snowballing resulted in an additional five articles. Four hundred and forty-seven duplicates were removed, and the titles and abstracts of the remaining 1129 articles were reviewed for eligibility, with 1014 articles ruled invalid and excluded. One hundred and fifteen full-text papers were evaluated using the predefined inclusion criteria and 39 articles did not fulfil the criteria and were excluded from the review. Thus, 76 articles met the inclusion criteria.

Figure 1. PRISMA flow diagram.
The 76 publications were distributed across 12 of the 50 countries in the sub-Saharan African region, of which 50% (n = 38) came from Eastern Africa, 36.84% (n = 28) from Western Africa, 10.53% (n = 8) from Southern Africa, and 2.6% (n = 2) from Central Africa (Fig. S1). Supplementary file 1 (Table S1) summarizes the key characteristics of the reviewed research articles. The findings revealed that 30.47% (12,858 of 42,202) cattle, 7.46% (603 of 8082) goats, and 13.81% (668 of 4838) sheep were infected with amphistomes (Table 1).
Table 1. Pooled prevalence of amphistome infections in domestic ruminants in sub-Saharan Africa based on different risk factors

Meta-analysis
The overall prevalence of amphistomes in domestic ruminants was 22% (95% CI, 10-37) (Fig. 2). The quality effects model revealed a significantly high heterogeneity in the studies included in the meta-analysis, (I² = 99%, P < 0.01), with Q=13862.01. This high heterogeneity confirms that the studies under this meta-analysis are from different populations. Publication bias analysis revealed asymmetric funnel plots (Supplementary file 2: Fig. S2) which indicated publication bias in the appraised studies. The results of the Egger’s test revealed a significant publication bias (P = 0.023), which is in line with the findings of the funnel plot. The imputed and observed effect sizes were found to be comparable by the Trim and Fill analysis results, and no studies were excluded from the study.
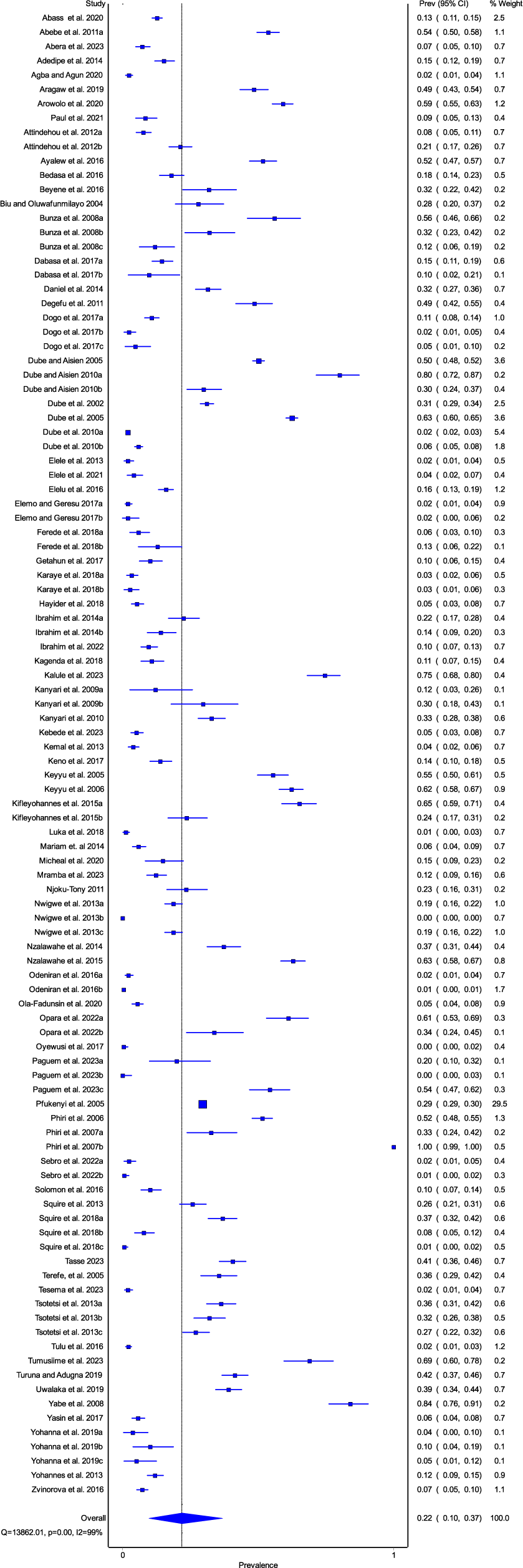
Figure 2. Forest plot for the prevalence of amphistome infections in domestic ruminants in sub-Saharan Africa.
Prevalence by region
The prevalence estimates from different regions are shown in Fig. 3a-d. The results of the study established that the southern region of Africa had the highest pooled prevalence of 25% (95% CI, 0-62) (Fig. 3c), followed by eastern and western Africa with 21% (95% CI, 14-29) and 21% (95% CI, 11-33), respectively (Fig. 3b, d). The least pooled prevalence of 16% (95% CI, 0-61) (Fig. 3a) was noted from central Africa. Results from the quality effects model revealed a high degree of heterogeneity between studies from all the regions with (I² =99%, P < 0.01) from eastern and western Africa; (I² =100%, P < 0.01) from southern Africa and (I² =98%, P < 0.01) from central Africa.

Figure 3. Forest plots of prevalence of amphistome in domestic ruminants from (A) central Africa, (B) eastern Africa, (C) western Africa, and (D) southern Africa recorded from 2002 to 2023.
Prevalence by host
The pooled prevalence of amphistome in cattle, goats, and sheep is shown in Fig. 4a-c. The highest prevalence was estimated in cattle 28% (95% CI, 12-47) followed by sheep 11% (95% CI, 4-20) and substantially lower for goats 5% (95% CI, 0-14). The quality effects model revealed a substantially high degree of heterogeneity between studies on different hosts, for cattle (I² =99%, P < 0.01) and for goats and sheep (I² =98%, P < 0.01).

Figure 4. Forest plots of prevalence of amphistome in (A) cattle, (B) goat, (C) sheep from 2002 to 2023.
Risk factors for infection
The sub-group on risk factors for infections in domestic ruminants is presented in Table 1. The quality effects model revealed a high degree of heterogeneity between studies from all sub-groups on risk factors infections in ruminants with I² >95% (P < 0.01) except for I² =89% (P < 0.01) on the medium body condition. The pooled prevalence for males at 32% (95% CI, 21-44) was higher than that of the females at 23% (95% CI, 13-36). Adult animals had a higher prevalence estimate at 37% (95% CI, 15-62) compared to young animals at 23% (95% CI, 5-52). The highest prevalence was estimated in the wet season at 36% (95% CI, 0-94) compared to the dry season at 21% (95% CI, 1-54). Animals with poor BCS had the highest prevalence estimate of 47% (95% CI, 34-60), followed by those with moderate BCS at 26% (95% CI, 13-42) and the lowest prevalence estimate was on animals with good BCS at 10% (95% CI, 1-25). The pooled prevalence measured by coprology 20% (95% CI, 6-39) was lower than the one measured at postmortem 23% (95% CI, 8-43). The years between 2002 and 2012 had a higher pooled prevalence of 29% (95% CI, 6-59) compared to 14% (95% CI, 10-19) noted between 2013 and 2023.
Heterogeneity and publication bias
The univariate meta-regression findings for the overall and subgroups for individual variables are displayed in Supplementary Table 2. Univariate meta-regression models identified three sources of variability in the prevalence of amphistome in domestic ruminants (P < 0.05). Condition factor had the greatest influence on heterogeneity, accounting for 60.1% of the variation. The heterogeneity of domestic amphistomosis was also explained by the differences in host and period in which studies occurred (P < 0.001), accounting for 15.6% and 14.8%, respectively. The analysis revealed that the period in which the studies were conducted significantly (P < 0.05) explains 21.6% and 27.7% of the variation in prevalence in the eastern and western regions, respectively. Furthermore, the two identified sources of heterogeneity for cattle, were study type and period (P < 0.001), which contributed 54.7% and 22.5% of the variation, respectively.
The combined variables had a substantial impact (P < 0.001) on the overall prevalence outcome accounting for 33.4% of the variation, with host and period (P < 0.001) as the major sources of heterogeneity (Supplementary Table 3). A combined meta-regression shows that all model predictors may significantly (P < 0.05) account for approximately 30.3% of the variability in the result in the eastern region and 27.9% in the western region. The only predictor variable that showed a statistically significant link with a P value less than 0.05 for both the eastern and western regions was the period as a factor. The combined variables in southern Africa accounted for 63.8% of the variability in the prevalence outcome, with a significant difference (P = 0.036). However, only the study type variable was statistically significant (P < .05). The prevalence of amphistome in cattle was significantly influenced by study type and period (P < .05) and all predictors together contributed 45.9% of the variation. However, for sheep and goats, the combined predictor factors had no significant effect on the prevalence outcome (P > .05). The combined factors significantly accounted for 34% and 30.6% of the variation in the prevalence outcome for age and sex, respectively. For the years 2002 through 2012 and 2013 through 2023, respectively, the combined variables explained 13.4% and 68.5% of the variation in the prevalence outcome.
Discussion
Despite the importance of amphistomes in livestock production, information on their prevalence in cattle, sheep, and goats is sporadic and limited in most sub-Saharan African countries, with data available from only 12 of the region’s 50 countries. Results demonstrated that more studies were conducted in the eastern region and western regions, which could be attributed to large number of livestock farmer practising extensive farming in these regions, and thus exposing animals to infection (FAO, 2017; Abera et al., Reference Abera, Kebede, Haile and Bekuma2023). The study found that the overall prevalence of amphistome in ruminants was lower in the most recent years (2013-2023). The decrease in the prevalence of amphistome infections can be attributed to farmers’ increased awareness of the risks associated with these parasites, improvements in the quality of veterinary services and management approaches, and improved hygiene and sanitation standards as proposed by Nyagura et al. (Reference Nyagura, Malatji and Mukaratirwa2024). Furthermore, differences in sample size, sampling processes, and diagnostic criteria can all have a significant impact on the results and contribute to the high heterogeneity. The considerable heterogeneity in the host subgroup may also be attributed to the various animal breeds examined (Kanyari et al., Reference Kanyari, Kagira and Mhoma2010; Pfukenyi & Mukaratirwa, Reference Pfukenyi and Mukaratirwa2018).
The estimated prevalence varies by region in sub-Saharan Africa with the Southern African region recording the highest pooled prevalence of amphistome infections, followed by the east and west, and central Africa with the lowest. The findings of this study are similar to those reported by Sibula et al. (Reference Sibula, Nyagura, Malatji and Mukaratirwa2024), who also recorded the highest prevalence rates of amphistome infections in wild ruminants from southern African countries. Pfukenyi & Mukaratirwa (Reference Pfukenyi and Mukaratirwa2018) proposed that the geographical distribution and prevalence of amphistome infections are influenced by the availability and abundance of susceptible definitive hosts. This may explain high amphistome infection rates in the east and west, where livestock populations are higher (FAO, 2017; Abera et al., Reference Abera, Kebede, Haile and Bekuma2023). However, Eduardo (Reference Eduardo1987) argued that the establishment of an amphistome species in a given region may be more dependent on the intermediate host than the final host. The variations in estimated prevalence between regions could be attributed to different climatic conditions (Gonzalez-Warleta et al., Reference Gonzalez-Warleta, Lladosa, Castro-Hermida, Martínez-Ibeas, Conesa, Munoz, López-Quílez, Manga-González and Mezo2013; Hajipour et al., Reference Hajipour, Mirshekar, Hajibemani and Ghorani2021), environmental conditions, ecology, host-parasite interaction, and collection season and management systems (Phiri et al., Reference Phiri, Chota, Muma, Munyeme and Sikasunge2011; Hajipour et al., Reference Hajipour, Mirshekar, Hajibemani and Ghorani2021; Tookhy et al., Reference Tookhy, Mahiza, Mansor, Yasmin, Ahmad, Hamzah and Idris2022). The regional variation in the prevalence rate of amphistome noted may also be influenced by the number of research undertaken in different regions. The prevalence of amphistome in domestic ruminants in central Africa may have been underestimated and cannot be relied on due to a paucity of studies conducted in the region.
The highest pooled prevalence was recorded in cattle, followed by sheep, whereas goats had the lowest. This was consistent with the findings by Rolfe (Reference Rolfe, Boray, Nichols and Collins1991), who reported that trematode infections were more frequent in cattle but less so in small ruminants. This could be explained by the varied feeding behavior of the animals (Mohammed et al., Reference Mohammed, Animut, Urge and Assefa2020), as cattle and sheep are grazers close to the ground, using their tongues to pull grass into their mouths or their lips and teeth to selectively consume vegetation close to the ground which subsequently makes them more susceptible to trematode infective stages (Yohanna et al., Reference Yohanna, Dung, Adejoh and Pam2019). Although the nibling tendencies of sheep (Mohammed et al., Reference Mohammed, Animut, Urge and Assefa2020) may decrease their exposure to aquatic vegetation contaminated with metacercariae, which play a crucial role in trematode life cycles. However, if the grazing environment contains infective trematode stages, such as metacercariae on the vegetation, sheep can still become infected (Kanyari et al., Reference Kanyari, Kagira and Mhoma2009). As observed in our results, Yusuf et al. (Reference Yusuf, Jima and Aseffa2024) also reported higher prevalence in sheep than goats and attributed the differences in grazing patterns between sheep and goats, which predisposed sheep more to the infective larval stages compared to goats. Goats are recognised for their browsing habits (Mohammed et al., Reference Mohammed, Animut, Urge and Assefa2020) which limit their exposure to infective stages of trematodes present near the ground (Tsotetsi et al., Reference Tsotetsi, Njiro, Katsande, Moyo, Baloyi and Mpofu2013). Dube et al. (Reference Dube, Masanganise and Dube2010) showed the same trend of sheep being better hosts than goats but indicated that goats develop better immunity than sheep which might also account for the differences in the parasite loads between them.
Several researchers have suggested that gender appears to have no bearing on infection allowances and propose that both males and females are equally prone to and vulnerable to infection (Keyyu et al., Reference Keyyu, Monrad, Kyvsgaard and Kassuku2005, Reference Keyyu, Kassuku, Msalilwa, Monrad and Kyvsgaard2006; Kanyari et al., Reference Kanyari, Kagira and Mhoma2009, Reference Kanyari, Kagira and Mhoma2010). Reviewed studies showed that males had greater pooled prevalence estimates than female hosts. This report contradicted other researchers who recorded significantly higher prevalence in females than males, which they attributed to the females’ immunological conditions during gestation and lactation, when they may be more susceptible to infection (Pfukenyi & Mukaratirwa, Reference Pfukenyi and Mukaratirwa2018; Zewde et al., Reference Zewde, Bayu and Wondimu2019). This was corroborated by Pfukenyi et al. (Reference Pfukenyi, Mukaratirwa, Willingham and Monrad2005), who reported a much higher prevalence in pregnant and lactating animals. However, the discrepancies observed in the reviewed studies could be attributed to differences in sample size, with more females being examined than male hosts.
The findings indicated that young animals were less likely to be infected than older animals. Similar observations were made where older animals were more susceptible to infection, and implying that age has a significant impact on the prevalence of trematodes (Aragaw et al., 2012; Zewde et al., Reference Zewde, Bayu and Wondimu2019). Pfukenyi & Mukaratirwa (Reference Pfukenyi and Mukaratirwa2018) attributed this to prolonged exposure in adults, which resulted in tolerance to the pathogenic effects of immature amphistomes while mature ones maintained their high egg production capacity. Furthermore, Zewde et al. (Reference Zewde, Bayu and Wondimu2019) noted that older animals are allowed to graze on pasture for longer periods, potentially contributing to a higher infection rate than young animals. Meguini et al. (Reference Meguini, Righi, Boucheikhchoukh, Sedraoui and Benakhla2021) hypothesized that amphistomiosis is more common in older cattle due to their reduced immune systems. Results showed that poor conditioned cattle had the highest overall prevalence which aligned with Kanyari et al. (Reference Kanyari, Kagira and Mhoma2010) report which found a link between low body condition and high amphistome prevalence in cattle based on coprology. Heavy infections are thought to cause weakness, repeated ruminal tympany, ruminal atony, weight loss, anaemia, and production losses (Anuracpreeda et al., Reference Anuracpreeda, Wanichanon and Sobhon2008; Pfukenyi & Mukaratirwa, Reference Pfukenyi and Mukaratirwa2018). Furthermore, Mpofu et al. (Reference Mpofu, Slayi, Mutero, Mlahlwa and Jaja2023) also indicated that parasitic infections can affect nutrient absorption and utilisation, potentially impacting overall growth. Analysis indicated that the host, period, and condition factors all significantly contributed to the heterogeneity in the prevalence. However, the variability became much more common when different factors are combined, indicating that understanding the interactions between these factors is crucial to understanding the complexity of epidemiological outcomes. The findings of this study support the notion that the epidemiology of amphistome parasites is usually caused by a confluence of factors that interact considerably rather than by a single determinant (Hajipour et al., Reference Hajipour, Mirshekar, Hajibemani and Ghorani2021). However, there was no observed relationship between body condition and amphistome infections in small ruminants because there was no data for meta-analysis.
The wet season was associated with higher pooled prevalence of amphistome infection in cattle compared to the dry season. Pfukenyi et al. (Reference Pfukenyi, Mukaratirwa, Willingham and Monrad2005) suggested that a high faecal egg counts during the wet season may be due to mature infections acquired during the preceding dry season. This observation contradicted other authors who noted the highest degree of parasite contamination in pasture occurs during the dry season (Chingwena et al., Reference Chingwena, Mukaratirwa, Kristensen and Chimbari2002; Phiri et al., Reference Phiri, Phiri, Chota and Monrad2007b). Pfukenyi et al. (Reference Pfukenyi, Mukaratirwa, Willingham and Monrad2005) further reported that only light infections are likely to occur since snail habitats and pastures are frequently inundated, flushing snails and free-living parasite stages out and disseminating them across a vast area. Mia et al. (Reference Mia, Hasan and Chowdhury2021) added that the extreme temperatures hinder the pathogenic phases of the parasite during the summer.
Meta analysis could not be performed at a species level, and this may be attributed to lack of prevalence statistics at the species level due to technical challenges in specific identification (Phiri et al., Reference Phiri, Phiri and Monrad2006). This was also consistent with Pfukenyi et al. (Reference Pfukenyi, Mukaratirwa, Willingham and Monrad2005), who asserted that specific species prevalence data are limited due to the difficulties in identifying amphistome to species level. The pooled prevalence in domestic ruminants measured by coprology was lower than the postmortem. This may be attributed to low sensitivity of coprological method as it only detects the presence of adult rumen fluke infection and infection with immature stages may have gone undetected (Malrait et al., Reference Malrait, Verschave, Skuce, Van Loo, Vercruysse and Charlier2015; Sargison et al., Reference Sargison, Francis, Davison, Barend, Handel and Mazeri2016). According to Horak (Reference Horak1971), one drawback of coprology diagnostic method is the high probability of finding few to no eggs in acute infections as they are usually associated with massive infection with immature flukes. Furthermore, Sargison et al. (Reference Sargison, Francis, Davison, Barend, Handel and Mazeri2016) also highlighted that although coprological technique/faecal egg count is the only practical test that is validated for diagnosis and identification of rumen fluke infections in live animals; it also has the potential to underestimate infections as it can only diagnose patent infections. Reviewed studies also showed a wider use of sedimentation technique to detect eggs in faecal samples. Although this technique has showed a huge success in large populations (Ibarra et al., Reference Ibarra, Montenegro, Vera, Boulard, Quiroz, Flores and Ochoa1998; Munguía-Xóchihua et al., Reference Munguía-Xóchihua, Ibarra-Velarde, Ducoing-Watty, Montenegro-Cristino and Quiroz-Romero2007), its lack of sensitivity in detecting low-intensity infections (Bosco et al., Reference Bosco, Ciuca, Maurelli, Vitiello, Cringoli, Prada and Rinaldi2023) often leads to misrepresenting the true prevalence. Bosco et al. (Reference Bosco, Ciuca, Maurelli, Vitiello, Cringoli, Prada and Rinaldi2023) proved that other techniques such as Mini-Flotac and Fluke Finder techniques should be used as they have shown to be more efficient, and sensitive compared to sedimentation.
For postmortem cases, histology and flattening were used to identify between species. However, these techniques need an expert skilled in identification of amphistome species identification of which they are scarce in the region (Lotfy et al., Reference Lotfy, Brant, Ashmawy, Devkota, Mkoji and Loker2010). Furthermore, determining the amphitsome species based only on morphology is difficult since the disease-causing flukes are mostly sexually immature (Chaoudhary et al., Reference Chaoudhary, Hasnani, Khyalia, Pandey, Chauhan, Pandya and Patel2015; Ikeuchi et al., Reference Ikeuchi, Kondoh, Halajian and Ichikawa-Seki2022). Thus, several researchers relied on the biased procedure of identifying a few adult worms that may be present in the rumen of animals (Horak, Reference Horak1971). Many errors could have been made in specific identification because the histology and flattening methods used are unreliable, according to Pfukenyi and Mukaratirwa et al. (Reference Pfukenyi and Mukaratirwa2018). Furthermore, Mitchell et al. (Reference Mitchell, Zadoks and Skuce2021) also highlighted that the morphologically plasticity of amphistomes result in numerous cases of misdiagnosis. As a result, polymerase chain reaction-based techniques providing rDNA Internal Transcriber Spacer (ITS-2) sequences have proven to be reliable tools to identify amphistome species and to determine their phylogenetic relationships (Itagaki et al., Reference Itagaki, Tsumagari, Tsutsumi and Chinone2003; Rinaldi et al., Reference Rinaldi, Perugini, Capuano, Fenizia, Musella, Veneziano and Cringoli2005).
Limitations of the study
Publication bias raised concerns regarding the reliability of the results and increases the risk of making poorly informed decisions about amphistomosis, even when the effect size remains unchanged when bias is considered. Therefore, it is critical that veterinary or departments monthly or yearly abattoir reports on amphistomosis in domestic ruminants are readily and easily accessible, and these should be incorporated in future research to improve our understanding of prevalence of amphistome in domestic ruminants.
Conclusion
The outcomes of this review and meta-analysis revealed that most sub-Saharan African countries have little to no information on the prevalence of amphistome in cattle, sheep, and goats, despite it being a substantial limiting factor in cattle and small ruminant production. Furthermore, the available data on amphistome infections in the region are often scattered across different studies and locations, making it challenging to obtain a clear picture of the overall status. This study highlighted that cattle were the most susceptible domestic ruminant. However, more epidemiological research on amphistomes is required in all sub-Saharan African countries to determine the true prevalence estimate in the region. The widely used coproscopic examination cannot be used for the early diagnosis of clinical amphistomosis which is vital for prompt treatment before considerable damages and economic losses are incurred. Developing diagnostic techniques capable of detecting prepatent infections in the final host will allow for a more accurate portrayal of the total prevalence of amphistome in African domestic ruminants. The findings of such research would provide vital information to aid in disease prevention, optimizing production efficiency to satisfy Africa’s rising population. Furthermore, additional research is needed to establish the economic significance of amphistomosis in domestic ruminants, as well as the efficacy of various anthelmintics. The high variation observed across and within subgroups emphasizes the need of using precise sample criteria to adequately integrate and quantify epidemiological data.
Supplementary material
The supplementary material for this article can be found at http://doi.org/10.1017/S0022149X24000725.
Declaration
The authors declare that there is no conflict of interest.
Author contribution
I.N. conceptualized the study under the guidance of S.M. and M.P.M. I.N. and P.I.N. developed the concept note, conducted the search, selected studies, and wrote the first draft of the manuscript under M.P.M.’s guidance. All authors contributed to the article, agreed on the final draft, and approved the submitted version.
Funding
No financial assistance was received for this study.







