1. Background on Mandated Shared-Decision-Making
As part of a move toward more patient-centered care, recent Medicare coverage decisions have included mandates to conduct shared decision-making. Shared decision-making is a process where clinicians partner with patients to ensure that patients’ values and preferences are integrated into well-informed decisions about their medical care. The goal of shared decision-making is to produce treatment decisions that satisfy the ethical goals of beneficence and respect for autonomy.Reference Moulton and King 1 There is evidence to suggest that shared decision-making processes, using tools such as decision-aids (DAs), can improve relevant domains of decision-making such as patients’ comprehension and risk perception.Reference Stacey, Legare, Lewis, Barry, Bennett, Eden, Holmes-Rovner, Llewellyn-Thomas, Lyddiatt, Thomson and Trevena 2 As a result, shared decision-making is widely-promoted across a range of clinical contexts. Since 2015, the Centers for Medicare and Medicaid Services (CMS) have mandated shared decision-making as a condition for reimbursement for 3 medical interventions. 3 Recent coverage updates suggest that CMS, and potentially other groups, are likely to expand these mandates to additional interventions over the coming years.Reference Jensen, Chin, Ashby, Hakmin, Fulton, Long, Farmer and Hutter 4
The core elements of shared decision-making are ethically unassailable, and substantive patient engagement in decisions should be promoted, particularly when those decisions involve important risk-benefit tradeoffs. However, the role of policy in mandating shared decision-making for specific procedures is less straightforward. Some scholars have framed mandated shared decision-making as an important policy lever because it has been shown to reduce invasive procedures, arbitrary variation in care and healthcare costs.Reference Oshima and Emanuel 5 These are important, and conventional, policy goals; however, they may be only contingently aligned with shared decision-making. The primary goal of shared decision-making is to promote high-quality decisions that effectively incorporate patients’ values and preferences. Shared decision-making’s success tends to be assessed by the extent to which goals such as improved patient comprehension about treatment options, patient engagement, or patient satisfaction are achieved. These goals are rarely questioned as desirable, but they are not typical policy targets. This exposes an important tension; there is no consensus regarding the specific decisions or contexts for which shared decision-making should be mandated or the metrics that should be used to define success or failure of these efforts. The CMS’s recent mandate involving primary prevention ICD implantation brings these questions regarding the role and goals of policy-mandated shared decision-making to the fore.
The primary goal of shared decision-making is to promote high-quality decisions that effectively incorporate patients’ values and preferences. Shared decision-making’s success tends to be assessed by the extent to which goals such as improved patient comprehension about treatment options, patient engagement, or patient satisfaction are achieved. These goals are rarely questioned as desirable, but they are not typical policy targets. This exposes an important tension; there is no consensus regarding the specific decisions or contexts for which shared decision-making should be mandated or the metrics that should be used to define success or failure of these efforts. The CMS’s recent mandate involving primary prevention ICD implantation brings these questions regarding the role and goals of policy-mandated shared decision-making to the fore.
2. Arguments in Favor of Shared Decision-Making for ICDs
There are strong reasons to support shared decision-making for ICD implantation. It is a somewhat unique intervention in that it represents a prophylactic surgical procedure that has been shown through randomized trials to improve mortality (for selected patients with heart failure) by preventing sudden cardiac death from ventricular arrhythmias.Reference Moss, Zareba, Hall, Klein, Wilber, Cannom, Daubert, Higgins, Brown and Andrews 6 However, ICD implantation does not improve quality of life or heart failure symptoms and carries peri-procedural risks and risk of late device malfunctions or infection. ICD therapy — either initial implant or continued therapy — may not be consistent with the goals of patients whose priority is quality rather than quantity of life. There are also data to suggest that ICDs are often misunderstood by patients and that the initial implantation decision is often mistreated by clinicians as a non-preference sensitive decision.Reference Hauptman, Chibnall, Guild and Armbrecht 7 Coupled with the significant costs associated with ICD implantation, these are all strong reasons to support increased adoption of robust shared decision-making, and this paradigm seems conceptually and ethically appropriate.
3. Problematic Factors of Mandated Shared Decision-Making for ICDs
The CMS mandate that physicians conduct shared decision-making using a DAReference Matlock, Varosy, Jenkins, Mellis, Vaorsy, Masoudi, Brega and Magid 8 for ICD implantation, however, raises key questions. First, the impact of shared decision-making processes (with or without DAs) on patients referred for ICD implantation is not well-known, and it is plausible that differences in implementation or changes in the way information is presented may have very different impacts on patients’ decisions, on clinical workflow or burden of compliance with the mandate.Reference Schenker, Fernandez, Sudore and Schillinger 9 Second, although patients sometimes fail to fully understand ICD therapy and its implications, this is the case for many other medical decisions as well.Reference Merchant, Dickert and Howard 10 This highlights the fact that it is not clear on what basis ICD implantation was specifically chosen for mandated shared decision-making.Reference O’Connor 11 Routine informed consent is considered sufficient for many other procedures in which tradeoffs exist between different options. The lack of clarity regarding the CMS mandate’s goal is exacerbated by the seemingly arbitrary nature of CMS’s decision to single out ICDs for mandated shared decision-making. Without articulated goals this mandate runs the risk of incentivizing perfunctory interactions.
The recent expansion of shared decision-making mandates and the case of ICD implantation demonstrates a need for a better understanding of what policy-mandated shared decision-making is designed to accomplish in order to judge when it should be required and how to judge its success. The following case study attempts to evaluate the impact of varying implementation strategies incorporating the DA in the shared decision-making interaction.
4. Case Study in Implementing the Shared Decision-Making Mandate for ICDs
In order to investigate implementation strategies for the new mandate, patients at 2 Emory sites were block-randomized in 1-month blocks to either receive an ICD DA 30 minutes prior to pre-implantation ICD consultation in the waiting room or at the end of the visit. The question of timing is important for two reasons. First, it is plausible that the DA could impact decision-making differently based on whether it is provided to a patient in advance of or after an encounter with a specialist. The former approach, for example, may help to educate patients in advance of an encounter so that they can formulate questions for the clinician but runs the risk of being somewhat acontextual. The latter may help patients to contextualize the discussion they have had and further educate themselves in deciding whether to go through with ICD implantation. Second, these two strategies differ logistically. Identifying appropriate patients ahead of a clinical encounter and providing the DA in advance requires administrative effort and coordination to screen clinic schedules and identify potential ICD candidates.
In our study, potential participants were identified by pre-screening clinic schedules. Enrolled patients were surveyed on the date of ICD implantation or, if they chose not to have an ICD implanted, by phone or mail using a survey adapted from an ongoing trial of shared decision-making for ICDs (NCT03374891). Surveys assessed outcomes including knowledge about ICDs, decisional conflict, and values-choice concordance. The study was approved by the Emory IRB, and consent was obtained for the survey.
During the study period, 42 patients evaluated in the clinic for primary prevention ICD implantation received the DA in block randomized fashion. Among these patients, 11 declined to complete the surveys, 4 were deemed not to meet criteria for ICD implantation after office consultation, and 3 were lost to follow up. Twenty-four patients completed the study, with 9 receiving the DA pre-visit and 15 at the end of the visit. Twenty-one patients had an ICD implanted, and 3 deferred an implantation. Of patients who chose not to undergo ICD implantation, 1 received the DA before and 2 received it at the end of the shared decision-making encounter. Baseline characteristics in both groups were similar. From a logistical perspective, screening clinic schedules and coordinating with implanting clinicians in order to distribute DAs pre-encounter took 1-2 hours per week.
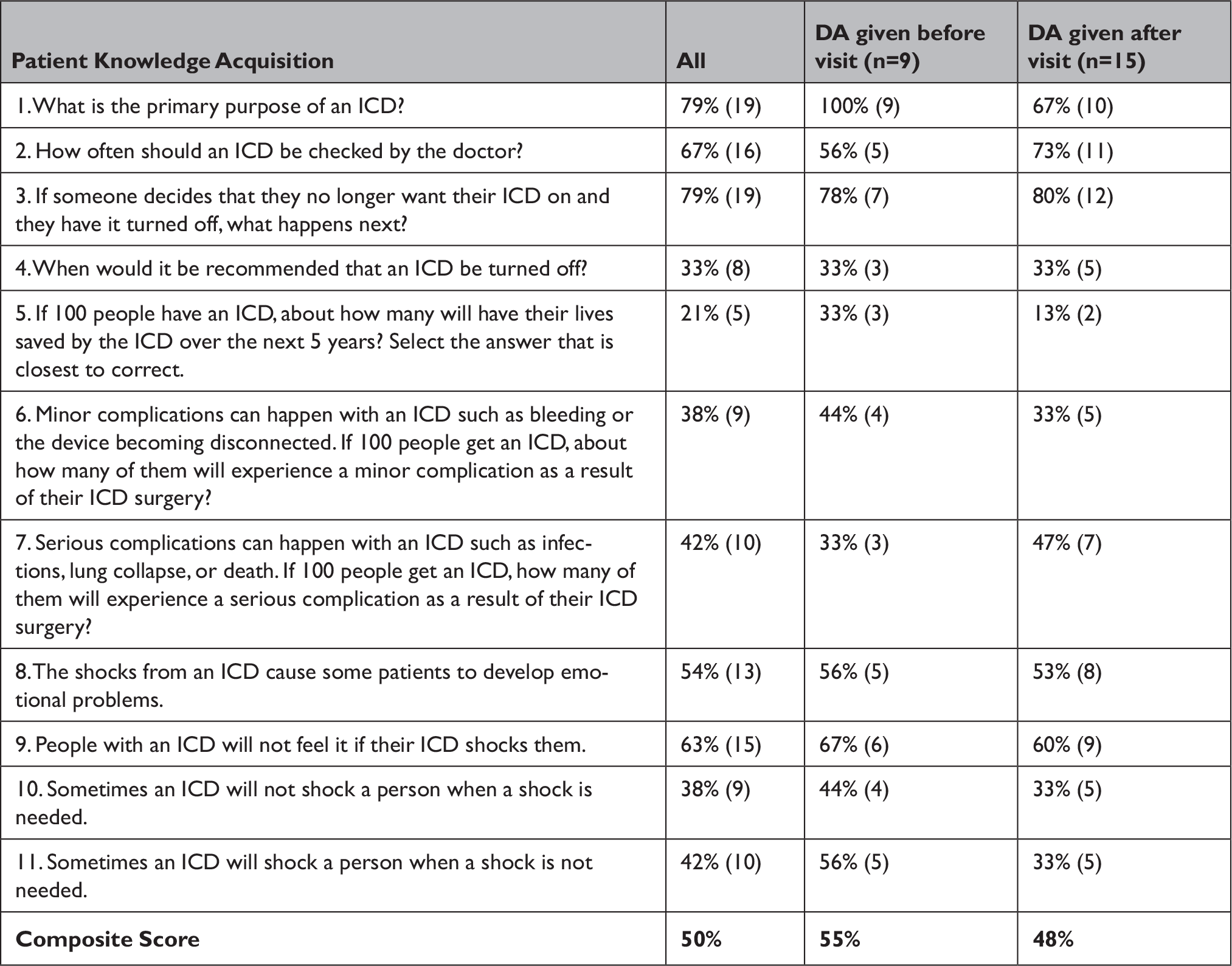
Overall knowledge regarding ICDs was similar between groups (Table 1); however, numerically more patients who received the DA beforehand understood the primary purpose of the ICD (9/9 vs. 10/15) and the risk of inappropriate shocks (5/9 vs 5/15). We did not identify any differences in values-choice concordance, decisional conflict,11 or reported patient engagement in the decision-making process based on DA timing.
Table 1 Percent of patients answering correctly on the ICD knowledge section of the survey, stratified by DA timing % correct (n)
5. Discussing the Lessons Learned Regarding Mandated Shared Decision-Making
This small pilot study was designed to evaluate how different implementation strategies may affect shared decision-making outcomes. It is presented here primarily as a case example. While it is not definitive, it demonstrates three ways in which implementation details matter and that clarity regarding core questions about policy-mandated shared decision-making is important. First, we observed a numeric difference in understanding of the purpose of the ICD between the two groups. This seems potentially important from an ethical perspective. Second, it suggests that differences (and potentially the impact of DAs overall) may not be in the actual choices that patients make. Third, it illustrates that different strategies have different impacts on clinical workflow and administrative burden. Identifying candidates for primary prevention ICDs by screening clinic schedules poses challenges. In our study, it took coordinators 1-2 hours per week, and almost 10% of screened patients were deemed not to be candidates for primary prevention ICDs after office consultation either because patients no longer met criteria for an ICD or were missing certain diagnostic tests. Thus, these data raise important questions about whether marginal potential improvements isolated to the domain of understanding are worth the administrative cost and whether the process or mandate are worthwhile if patients’ decisions do not appear to be impacted.
This experience demonstrates the need to be clear about the goals of the shared decision-making mandate and the metrics by which this policy and approaches to implementation should be assessed. Contextualizing these data and evaluating policy-mandated shared decision-making require addressing three important and interconnected questions: 1) what is the primary goal of the policy; 2) which metrics should be prioritized; and 3) what are the challenges related to its implementation?
If the goal of the mandate is primarily to improve patients’ understanding of the medical treatment options and engagement in the decision-making process, then assessments of success should focus on metrics such as comprehension of the therapy, patient engagement, or satisfaction, and decision conflict. These are primarily ethically-driven goals that advance patient autonomy and beneficence. They also advance transparency and may promote trust. Improvements in metrics such as decision conflict, patient engagement, and patient comprehension can be detected through surveys and interviews with patients and clinicians and through direct observation. Demonstrated improvements in these domains would define success and help to identify the best approaches among different implementation strategies.
There are challenges to justifying adoption of these ethically-driven outcomes as the goal of a policy mandate. Perhaps most obviously, implementation strategies that impact these outcomes can impact other processes of care and may involve resource tradeoffs. In our small study, providing the DA to patients earlier had a signal of improvement in patients’ understanding of the purpose of ICDs. However, this came at the cost of increased administrative burden in terms of identifying appropriate patients and distributing the information. No impact was observed on other shared decision-making domains or patients’ choices. Clinicians and payers must assess whether the impact on experiential domain alone is sufficient to justify the burden of shared decision-making. This is not to trivialize these benefits. However, they are not typical policy targets. In most cases, policy tends to focus on decreasing low-value care, advancing public health initiatives, and improving patient safety and outcomes. An assessment of the yield and costs is thus particularly important. Moreover, while improving patients’ understanding may be a reason to consider requiring shared decision-making, CMS has not stated that this is a primary goal of shared decision-making for ICDs or presented evidence regarding the particular implications of patients’ lack of understanding in the context of ICDs. The latter is important in distinguishing why this decision would be prioritized for policy-mandated shared decision-making when gaps in patient understanding about procedures in general are common.
Furthermore, the content of the DA is the focus of current measurements of patients’ understanding of ICDs, but this provides an incomplete assessment of patient comprehension of what is at stake. The DA cited in the CMS mandate describes a uniform set of risks and benefits of an ICD, without integrating patient specific factors. The practical reasons for this are obvious, but patient-specific factors are important to consider when choosing whether to have an ICD. Even among patients who meet criteria for an ICD, national registries show significant heterogeneity in the degree of benefit patients get from an ICD. For instance, patients who are older, have multiple comorbidities, and are frail are at higher risk of a non-arrhythmic death (i.e. no benefit from an ICD) and have higher procedural complications. For this subset of patients, the strength of a recommendation to implant an ICD, even if the patients’ goals are to prolong life, are less clear. Thoughtful clinicians will use the DA as a starting point and explain to patients that their risks and benefits may be more or less. However, this may complicate assessments of patients’ understanding of these nuanced issues and the impact of DAs on that understanding.
If the primary goal of mandated shared decision-making for ICDs is to increase alignment between patients’ values and their choices, an importantly different set of metrics must be targeted to measure success. Values-choice concordance is much a more stringent metric, because it requires the policy or practice to demonstrate an impact on actual decision-making. Assessing values-choice concordance is difficult in the context of an individual decision but can be measured across a population by assessing what matters most to patients and examining the apparent alignment between the distribution of stated values and treatment decisions. In the case of ICDs, an intervention would be successful if it led to a decline in ICD implantation among patients whose values do not align with therapies focused on maximizing lifespan without improving quality of life. This metric has previously been advocated for policies aimed at improving decision quality.Reference Sepucha, Fowler and Mulley 12 Moreover, shared decision-making with DAs has been shown to improve values-choice concordance for cardiac procedures such as left ventricular assist device implantation.Reference Allen, McIlvennan, Thompson, Dunlay, LaRue, Lewis, Patel, Blue, Fairclough, Leister, Glasgow, Cleveland, Phillips, Baldridge, Walsh and Matlock 13 On the other hand, the impact of shared decision-making on the choice of anticoagulation for atrial fibrillation appears negligible.Reference Kunneman, Branda, Hargraves, Sivly, Lee, Gorr, Burnett, Suzuki, Jackson, Hess, Linzer, Brand-McCarthy, Brito, Noseworthy and Montori 14 It is unclear at this stage whether DAs achieve this goal in the context of ICD implantation.
There are several challenges associated with mandating shared decision-making with the goal of improving values-choice concordance. First, it is difficult to determine empirically whether shared decision-making meets the goal. It is insufficient to use the rate of ICD implantation before and after a mandate to assess success. Promoting values-choice concordance could systematically shift which patients decide to have an ICD implanted but register a small change in the overall rate of implantation. Similarly, the overall effect could be an increase or decrease in the rate of implantation; a change in either direction could be desirable. The appropriate rate of ICD implantation depends on the distribution of values or preferences, and sophisticated assessments are necessary to make the determination about whether the impact of shared decision-making with DAs is positive. A second challenge is that shared decision-making during ICD implantation may impact patients’ downstream choices, which may be difficult to capture. Though the shared decision-making mandate describes a singular interaction, shared decision-making does not need to be an isolated event. Eliciting patients’ values during the consultation for ICD implantation may facilitate future shared decision-making opportunities.Reference Knoepke, Allen, Kramer and Matlock 15 For example, in the case of ICDs, one motivation to improve upfront decision-making is to decrease the extent to which patients in hospice care have active ICDs because they never understood that the device could be deactivated if therapy no longer aligns with their goals. In the case of generator exchange decisions after a battery is depleted, many patients may fail to appreciate that this is elective, and their goals or prognosis may have changed appreciably since the initial ICD implantation. Though unknown, it is plausible that improved understanding at the time of initial implant may help to reduce therapeutic inertia and facilitate more concordant decisions when exchange is considered. In short, accounting for long-term impacts is critical to evaluating the success of this type of mandate.
The goal of improving values-choice concordance for ICD decisions also relies, to some extent, on an assumption that patients have strong, pre-formed preferences between quality of life and quantity of life. However, many patients do not and appropriately care about both. The absence of strong authentic preferences has been illustrated in the context of advanced directives, for example.Reference Halpern, Loewenstein, Volpp, Cooney, Vranas, Quill, McKenzie, Harhay, Gabler, Silva, Arnold, Angus and Bryce 16 In this context, preferences and weighing of values are often shaped in the context of discussions with clinicians and are heavily influenced by choice architecture. It is likely the case that there is a similar absence of pre-formed views regarding quality/quantity tradeoffs in the context of ICD implantation, and this makes the assessment of values-choice concordance challenging.
Despite these complications, focusing on values-choice concordance does help to address two of the challenges raised by approaches grounded in patients’ comprehension or engagement. First, it provides a clear basis for identifying which decisions to prioritize. Specifically, policy-mandated shared decision-making should be considered when there is significant evidence of values-choice discordance at baseline and evidence that shared decision-making processes help to correct this misalignment. Second, the value proposition of the policy is clearer, because substantively different choices are made if the intervention is successful. As noted, however, more robust data are necessary to justify the policy’s enactment and maintenance.
We have identified a set of potential goals of mandated shared decision-making in the context of ICD implantation and have argued that clarifying the goals of the policy is critical, but it is important to clarify that these goals are not mutually exclusive. Improving ethically-driven, experiential outcomes while increasing alignment between patient values and their choices may be achievable. It may also be that aligning choices with values reduces total costs.
Finally, shared decision-making may be promoted or required to reduce costs or practice variation or improve health outcomes. These goals are traditional, often uncontroversial, targets for health policy interventions. Though they are not inherently patient-centered, they are important, and there may be instances where shared decision-making plausibly impacts these outcomes. For some elective procedures, for example, unexplained variations or excessive utilization among subsets of the population may be both unjustifiable and likely explained by different forms of patient engagement and decision-making. Shared decision-making using DAs may be an attractive strategy for addressing these challenges, particularly because it relies on patient engagement — which everyone agrees should generally be promoted — rather than imposition of restrictions or other barriers to curb inappropriate use.
While reductions in cost or improvements in certain health outcomes are desirable, there are important problems with their use as reasons to require — or metrics for evaluating — policy-mandated shared decision-making for ICDs. Most directly, ICDs reduce mortality, are cost effective, and are recommended by multiple society guidelines for eligible patients. Despite reducing cost, mandated shared decision-making would harm patients if it reduced implantation in patients for whom the therapy is likely to be beneficial and who have values consistent with implantation. Similarly, a focus on health outcomes could be problematic; shared decision-making may appropriately lead some patients who could benefit from ICD therapy to decline implantation based on their values. In this context, overall survival in the population may decrease. If these choices are more informed and congruent with patients’ values because of properly functioning shared decision-making, this outcome should not be considered a failure. Because these goals are only indirectly connected to shared decision-making itself, they raise real challenges as a basis for requiring shared decision-making or evaluating its success.
If CMS had clearly outlined the basis for choosing ICDs for mandating shared decision-making, its implementation could be studied and refined to support the agency’s goal. Shared decision-making solutions should focus on addressing a “problem” specific to context. Regarding ICD decisions, the CMS has not appropriately defined the scope of the “problem” or the anticipated outcomes that shared decision-making is intended to achieve. In the absence of formal guidance from the CMS and no published clinical trials studying, to date, strategies for shared decision-making in the context of ICDs, clinicians are likely to engage in a heterogenous set of practices designed primarily to meet whatever is minimally required to qualify for a shared decision-making interaction. As illustrated by our case example, we think it is important and helpful to gather rigorous data on the impact of various implementation strategies. We also encourage the CMS to consider emerging evidence to provide guidance for clinicians and health systems and to continue to clarify the underlying goals of the mandate so that the most productive strategies can be identified and promoted.
Conclusion
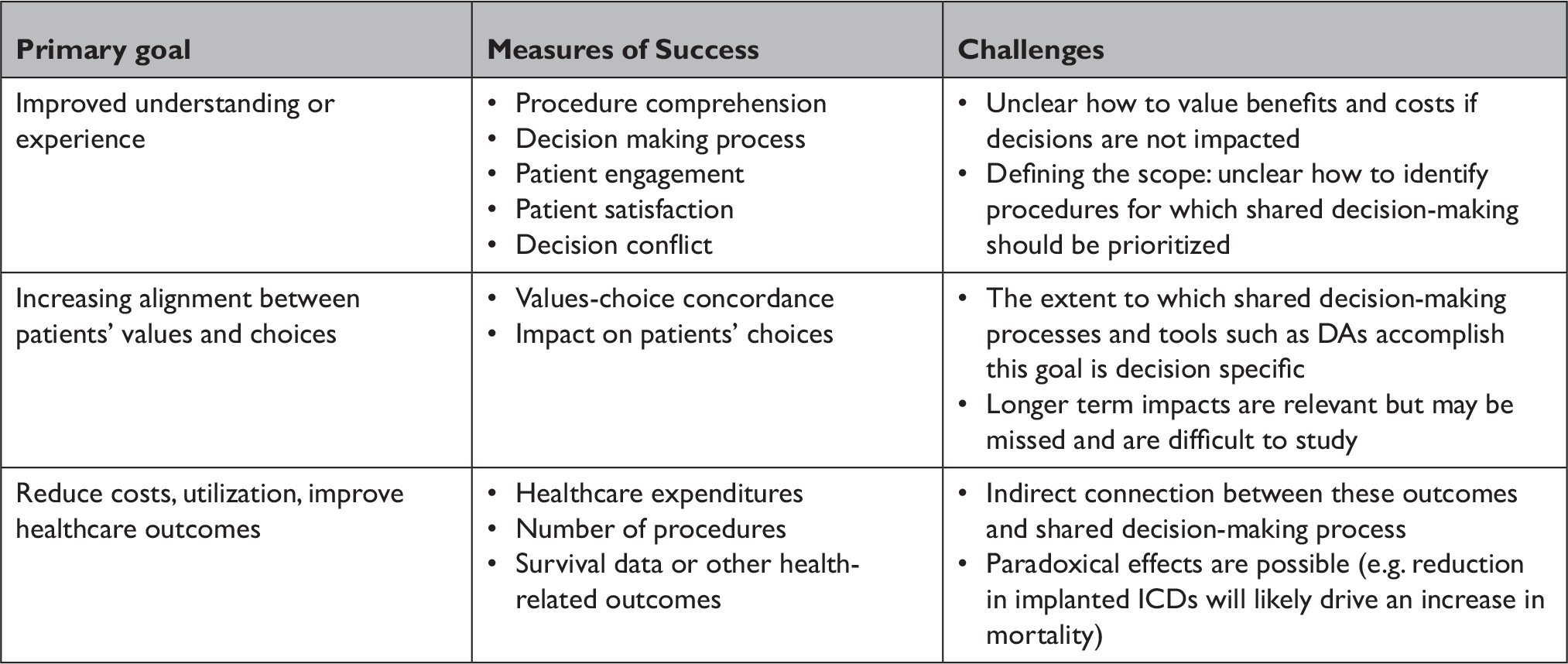
As the CMS appears poised to extend mandated shared decision-making to other realms, lessons learned from the shared decision-making mandate for ICDs are critical to consider. Shared decision-making is an important patient-centered strategy with solid ethical groundings. When there are important tradeoffs associated with clinical decisions, it is generally the “right thing” to do. However, policy mandated shared decision-making itself is not a panacea. Clearly specifying the goals of the mandate and the intended outcome can assure that policy mandated shared decision-making actually improve patient centered care and do not generate perfunctory interactions. The need for specificity in policy mandated shared decision-making for ICDs and other interventions is particularly important different shared decision-making processes can involve real tradeoffs (Table 2). These may include personnel or administrative costs, as well as potential unintended effects on decisions themselves. Specifying the primary goal of policy-mandated shared decision-making can help to define when shared decision-making is a particularly effective tool, can provide a framework for ascertaining whether the policy is successful, and can provide guidance on refinement of implementation strategies.
Table 2 Potential goals, measures of success, and challenges of policy-driven shared decision-making
We have identified a set of potential goals of mandated shared decision-making in the context of ICD implantation and have argued that clarifying the goals of the policy is critical, but it is important to clarify that these goals are not mutually exclusive. Improving ethically-driven, experiential outcomes while increasing alignment between patient values and their choices may be achievable. It may also be that aligning choices with values reduces total costs. Moving forward, a clearly articulated policy goal would help guide implementation researchers and clinicians alike to design and study strategies to optimize shared decision-making outcomes and avoid unintended consequences. The latter goal of avoiding unintended consequences is particularly salient in the context of recent policy mandates such as “hotspotting” and readmission reduction efforts.Reference Finkelstein, Zhou, Taubman and Doyle 17
Acknowledgements
The authors wish to thank Dr. Leon Darghosian for his help in conducting screenings and administering the surveys used in this study.
Note
Funding was received from the Institutional Synergy Award through the Woodruff Health Sciences Center at Emory University. All aspects of the study were approved by the Institutional Review Board of the Emory University. Confidentiality safeguards were affirmed, and written informed consent was obtained from all participants. Dr. Dickert reports receiving research funding from Agency for Healthcare Research and Quality, National Institutes of Health, Patient-Centered Outcomes Research Institute, and the Greenwall Foundation. Dr. Rao is supported in part by the Bryon Williams Jr., M.D. Fellowship Fund, the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR002378 and TL1TR002382, and the Agency for Healthcare Research and Quality Award Number 1F32HS028558.




