Published online by Cambridge University Press: 20 June 2019
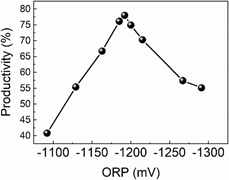
The physicochemical properties and broad applications of graphene have been extensively studied, but its preparation method is still a bottleneck, and it cannot simultaneously meet the requirements of low process cost and high quality of products in the time being. In this article, the redox potential was employed to control the quality of graphene prepared from graphene oxide by chemical reduction. The effects of the initial redox potential on the productivity, microscopic morphology, and structural and intrinsic properties of graphene were investigated. Results showed that there was an optimum initial redox potential range between −1200 and −1180 mV. In such a range could the graphene with a high yield be obtained, and layers of graphene products could be stabilized at 1 or 2 layers. Therefore, the redox potential could be used as an effective parameter instead of trying to design orthogonal tests to determine the optimal conditions and control the synthesis of graphene.