Published online by Cambridge University Press: 02 May 2017
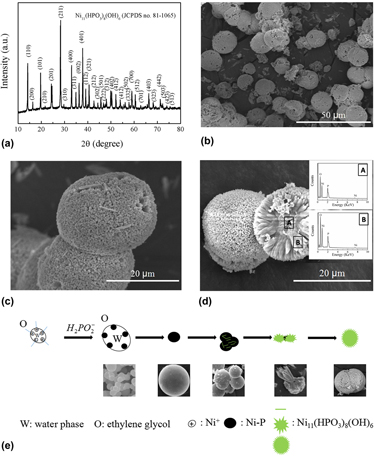
Due to the increasingly severe environmental pollution problems, the development of semiconductor photocatalysts is being extensively carried out because they have exhibited high activities for the degradation of many organic and inorganic pollutants. In this study, waxberry-like Ni11(HPO3)8(OH)6 microball photocatalysts with a diameter of 10–20 μm have been successfully synthesized via a solvothermal route by using NiSO4 as a Ni-source and NaH2PO2 as a P-source in a mixture of ethylene glycol and water under the optimized conditions with a Ni:P molar ratio of 1:2 at 200 °C for 16 h. The as-prepared photocatalysts were characterized by powder X-ray diffraction, scanning electron microscopy, energy-dispersive X-ray analysis, Brunauer–Emmett–Teller N2 adsorption, zeta potential, ultraviolet-visible (UV–vis) absorption, and photocatalysis tests. The decolourization of organic dyes, methylene blue and rhodamine B, under ultraviolet light over the as-prepared products reveals an excellent photocatalytic performance due to the good absorption for ultraviolet light.
Contributing Editor: Akira Nakajima