Article contents
Effect of Al and Ag addition on phase formation, thermal stability, and mechanical properties of Cu–Zr-based bulk metallic glasses
Published online by Cambridge University Press: 27 April 2011
Abstract
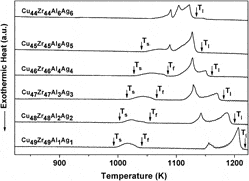
The compositional dependence of phase formation, thermal stability, and mechanical properties of (Cu0.5Zr0.5)100−x(Al0.5Ag0.5)x (x = 2, 4, 6, 8, 10, 12, 14, 16) bulk metallic glasses was studied. The Young’s modulus (85 ± 1 to 95 ± 1 GPa) and Vicker’s hardness (585 ± 7 to 627 ± 8 Hv) increased with increasing Al + Ag content from 8 to 16 at.%, respectively. The liquidus temperature decreased from 1210 ± 2 to 1110 ± 2 K with increasing Al + Ag content from 2 to 16 at.%. The starting temperature of the endothermic event related with transformation of the low-temperature equilibrium phases to CuZr parent phase increased from 997 ± 2 to 1043 ± 2 K, whereas the electronegativity difference for the (Cu0.5Zr0.5)100−x(Al0.5Ag0.5)x (x = 2, 4, 6, 8, 10, 12) alloys decreased from 0.2838 to 0.2713. The martensitic transformation temperatures decreased with increasing Al and Ag content for the (Cu0.5Zr0.5)100−x(Al0.5Ag0.5)x (x = 2, 4, 6, 8) alloys.
Information
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 2011
References
REFERENCES
- 9
- Cited by

