Published online by Cambridge University Press: 18 April 2017
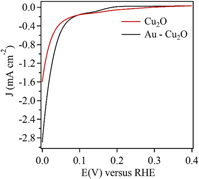
Copper(I) oxide (Cu2O) is a very favorable p-type semiconductor. It is an appealing candidate for photoelectrochemical water splitting. Here we report the fabrication and performance of gold (Au) underlayer–Cu2O composite photocathode for photoelectrochemical water splitting. The composite photocathode was fabricated by the electrodeposition technique. The different morphologies of the Au underlayer were achieved via variation in the process parameters including applied potential, electrolyte pH, and the presence of L-cysteine in the electrolyte. The Cu2O overlayer was also deposited using electrodeposition. Additionally, the influence of morphology variation, of the Au underlayer, on the performance of the composite photocathode was evaluated. It was observed that the performance of the composite photocathode increased by 81% when compared to a control sample of Cu2O. The composite photocathodes were characterized by scanning electron microscopy, X-ray diffraction, and electrochemical impedance spectroscopy.
Contributing Editor: Xiaobo Chen