No CrossRef data available.
Published online by Cambridge University Press: 18 October 2019
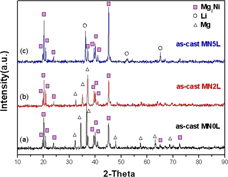
Lithium was added to the hypereutectic Mg–Ni alloy to investigate the effect of volatilization of Li on the hydrogen storage characteristics of the eutectic Mg–Ni alloy at 300 °C. After fully activated at 300 °C, Li was almost completely volatilized and the structure of Li-containing Mg82Ni18 alloy was converted to the structure of Li-free Mg82Ni18 alloy, but hydrogen absorption capacity significantly decreased. This is because volatilization of Li weakened the bonding between eutectic Mg and Mg2Ni, lowering the catalytic effect of Mg2Ni on Mg. The decrease in hydrogen absorption capacity was more obvious with increasing Li content. In addition, experimental alloy in powder form could increase surface area, causing Li to volatilize at 300 °C.
Present Address: Army Academy R.O.C., Jhongli District, Taoyuan 32092, Taiwan.