No CrossRef data available.
Published online by Cambridge University Press: 09 May 2017
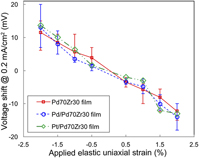
In this study, the influence of an externally applied elastic strain on the electrochemical activity of metal film catalysts during the oxygen reduction reaction (ORR) was examined. A novel three-layer specimen, composed of a 10 nm-thick Pt or Pd surface film on a 20 nm-thick Pd70Zr30 metallic glass film that was first deposited on a polymer substrate was used. The intermediate metallic glass layer is instrumental in allowing the top-layer catalytic film to be elastically deformed to a large elastic strain, (up to 2%), enabling a strain effect to be clearly observed. The results consistently show that an applied compressive strain improves the ORR catalytic activity of the Pd and Pt surface layer, while a tensile strain degrades it. These experimental findings are consistent with the prediction of the d-band model, and provide an opportunity to improve the catalytic response during ORR.
Contributing Editor: Edward M. Sabolsky