Published online by Cambridge University Press: 29 December 2016
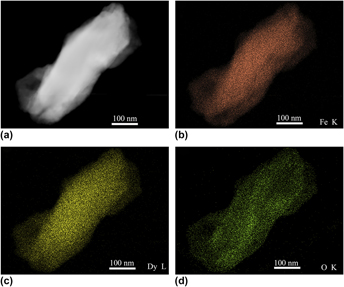
Ball milling induced the formation of nanocrystalline and amorphization phase in Fe–25.68% Dy2O3 powder mixtures (mass fraction, %). The microstructure was investigated by using X-ray diffraction and transmission electron microscopy. The transformation of Dy2O3 from cubic to monoclinic crystal structure and then to the amorphization was observed during ball milling. A few Dy atoms were dissolved into Fe crystal structure, which was discussed using mechanical kinetics. After 48 h of ball milling, the homogenous mixtures of supersaturated nanocrystalline solid solution of Fe (Dy, O) and Dy2O3 amorphization were formed and the elements of Fe, Dy, and O were distributed uniformly in the ball-milled particles. During the whole ball mining process, a rapid decrease in Fe grain size was observed over the initial time period, while a constant value was presented in later stage, resulting in a final size of about 20 nm. The mechanism of the microstructural evolution of powder mixtures was analyzed and discussed.