No CrossRef data available.
Published online by Cambridge University Press: 17 January 2011
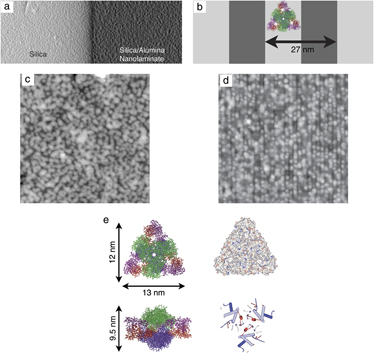
Technological progress in the synthesis and characterization of nanometer-scale structures has improved understanding of molecular and colloidal aggregation, self-assembly, and crystal growth. While substrates are commonly used to control nucleation and growth in metal and semiconductor crystals, their use in protein epitaxy has been limited by the lack of substrate structures commensurate with protein sizes. In this paper we describe the use of polished cross sections of amorphous alumina–silica nanolaminates whose periods varied from 8 to 200 nm in the formation of self-assembled monolayers of the protein macromolecule aspartate transcarbamoylase (ATCase). Scanning force microscopy images of rapidly deposited ATCase demonstrates one-dimensional protein ordering along 13.5 nm wide silica nanolaminate. Numerical studies of irreversible adhesion indicate that patterning can induce a higher degree of ordering by varying the substrate periodicity. We expect this to have implications for nucleation and growth of both two-dimensional crystalline layers and bulk protein crystals.