Published online by Cambridge University Press: 03 March 2016
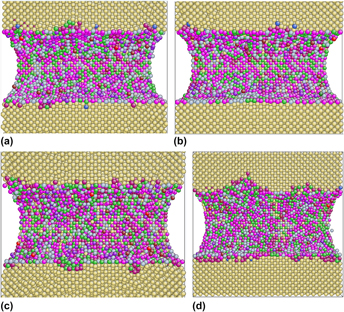
Evidences show that the composition of β″ formed in age hardening of Al alloys should be the prototype Mg5Si6 with Al and/or Cu addition. In the present work, molecular dynamics simulations are carried out to investigate the influence of the addition of Al and/or Cu to the mechanical properties of the prototype Mg5Si6. Our simulations imply that Mg5Si6 with both Al and Cu addition has relatively poor mechanical performance when compared with other three models. The snapshots of atomic configurations during uniaxial tension test illustrate that only if both Al and Cu dissolve in β″, clusters can form through Al atoms segregating around Cu atoms, thus applying different stress fields on the Al matrix, resulting different mechanical properties in comparison with other three β″ models.