Article contents
Phase selection of undercooled solidification of Ni–4.5 wt% B alloy
Published online by Cambridge University Press: 20 December 2013
Abstract
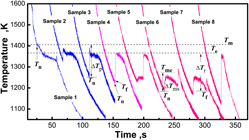
Solidification of undercooled Ni–4.5 wt% B alloy melt was investigated by glass fluxing and cyclic superheating. A maximum melt undercooling up to ΔTp = 283 K has been achieved. If ∆Tp < 175 ± 10 K, the primary solidification is L → Ni3B; the structure consists of Ni3B dendrite + lamellar eutectic; the phase sizes and fractions depend on ∆Tp. If ∆Tp ≥ 175 ± 10 K, the primary solidification is L → Ni/Ni23B6; the structure consists of the dot-phase region + the anomalous eutectic/network boundary; the phase fractions mainly depend on ∆Tr; the dot phases are determined as rod eutectic and dot precipitates, while the network boundary is the divorced eutectic. The solidification pathways show that there is a common critical nucleation temperature, 1227 ± 10 K, for metastable eutectic reaction in hypoeutectic and hypereutectic Ni–Ni3B alloys.
Information
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 2013
References
REFERENCES
- 7
- Cited by

