No CrossRef data available.
Published online by Cambridge University Press: 20 November 2015
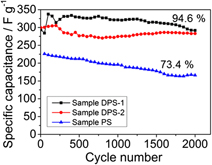
Developing high energy density supercapacitors is of great importance to the transportation, consumer electronics, and micro-grid energy storage sectors. Recently, the development of high voltage organic electrolyte based supercapacitor devices has been gaining much attention. Among them, there is an on-going intense interest in investigating high capacity lithium ion storage anode materials in hybrid supercapacitors. However, developing high capacity cathode materials for high voltage organic electrolyte supercapacitor devices is rarely investigated. The low electrical double layer capacitances of carbon cathode electrodes, which are widely used in current supercapacitor devices, are often the limiting bottleneck. In this contribution, we investigated the electrochemical energy storage behavior of a polyaniline (PANI)-single wall carbon nanotube (SWCNT) composite material in an organic electrolyte as a supercapacitor cathode. The PANI-SWCNT composite exhibits a high specific capacitance of 503 F/g, of which 58.8% of the total capacitance is attributed to the pseudocapacitive and electrical double layer energy storage. The cycling stability of the PANI-SWCNT composite could be further improved by polydopamine (PDA) modification. The PDA with strong adhesion properties is able to prevent mechanical degradation. The PDA modified PANI-SWCNT shows excellent stability with only 5% degradation after 2000 cycles.