Article contents
Red blood cells membrane vehicle co-delivering DOX and IR780 for effective prostate cancer therapy
Published online by Cambridge University Press: 20 October 2020
Abstract
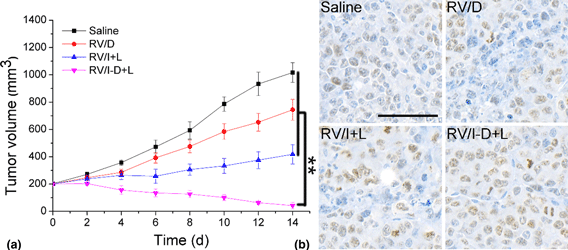
The cellular accumulation of drug delivery systems (DDSs) is a critical parameter to determine the final outcome of cancer chemotherapy. Herein, we designed a red blood cells membrane-based vehicle (RV) and employed it to load both doxorubicin (Dox) and IR 780 (RV/I-D). The photothermal-assisted chemotherapy efficacy of RV/I-D on the treatment of cancer was tested on a prostate cancer model. Excitingly, the results showed that RV/I-D was stable and safe nanoparticles with size at about 100 nm. Moreover, upon the increase of system temperature using photothermal effects of IR780, the drug release of the DDS was accelerated. Above all, the DDS also increased the accumulation of drugs into the Dox-resistant prostate cancer cells (PC-3/Dox) both in vitro and in vivo and showed enhanced anticancer performance.
Keywords
- Type
- Article
- Information
- Copyright
- Copyright © The Author(s), 2020, published on behalf of Materials Research Society by Cambridge University Press
References
- 6
- Cited by



