Article contents
Structure and electrochemical performance of LiFePO4 modified with mononuclear and binuclear phthalocyanines as cathode materials
Published online by Cambridge University Press: 09 February 2017
Abstract
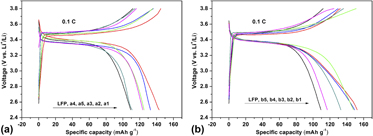
Two series of lithium iron phosphate (LiFePO4) nanocomposites are prepared by a solvothermal method coupled with high temperature calcination using mononuclear and binuclear metal hexaaminophthalocyanines as modulatory additives, respectively. Physical and electrochemical performances of the composites as cathode materials of lithium-ion batteries are characterized by inductively coupled plasma (ICP), X-ray diffraction (XRD), infrared (IR), scanning electron microscopy (SEM), transmission electron microscopy (TEM), and electrochemical techniques. The results indicate that the as-synthesized samples modified with binuclear metal phthalocyanines can improve electrochemical properties of LiFePO4 (LFP) for lithium-ion batteries. The composite using binuclear manganese hexaaminophthalocyanine as additive can achieve the highest initial specific discharge capacity of 152.3 mAh/g at 0.1 C, higher than that of ones modified with the corresponding mononuclear phthalocyanine 143.0 mAh/g. Furthermore, the most excellent product exhibits a pretty good capacity retention of 93.0% after 50 cycles at 0.1 C, cycling stability, and low charge transfer resistance of 58.7 Ω.
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 2017
Footnotes
Contributing Editor: Chongmin Wang
References
REFERENCES
- 3
- Cited by



