Published online by Cambridge University Press: 27 July 2015
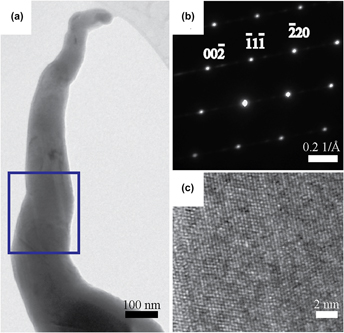
Single-phase crystalline ZnSnP2 nanowires have been prepared via simple chemical vapor deposition method using powdered Zn and SnP3 as the precursors in a custom-built tube furnace reactor. The sublimed precursors were allowed to react with thermally evaporated Sn nanoparticles to yield ZnSnP2 nanowire films over areas of 40 mm2. The cumulative observations suggest that the Sn nanoparticles served both as the growth seed and main contributor of Sn. Prolonged growth time favored formation of Zn3P2 nanowires when the Sn supply was exhausted. For optimal growth conditions, surface and bulk elemental analyses showed homogenous elemental distribution of Zn, Sn, and P, with chemical composition close to 1:1:2 stoichiometry. Powder x-ray diffraction data and Raman scattering of the nanowire films along with single-nanowire analysis using high-resolution transmission electron microscopy indicated that the as-prepared ZnSnP2 nanowires possessed a sphalerite crystal structure, as opposed to the antisite defect-free chalcopyrite structure. Photoelectrochemical measurements in aqueous electrolyte showed that the as-prepared ZnSnP2 nanowires are capable of sustaining stable cathodic photoresponse under white light illumination. Overall, this study presented a benign and straightforward approach to prepare single-phase Zn-based phosphide nanowires suitable for energy conversion applications.