Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by
Crossref.
Cai, Huisheng
Zhao, Zheng
Wang, Qudong
Zhang, Nannan
and
Lei, Chuan
2022.
Study on solution and aging heat treatment of a super high strength cast Mg-7.8Gd-2.7Y-2.0Ag-0.4Zr alloy.
Materials Science and Engineering: A,
Vol. 849,
Issue. ,
p.
143523.
Liu, Zhenyang
Pei, Zongrui
Zhou, Nan
Zheng, Kaihong
and
Chen, Bin
2023.
The γ” Phase in Mg-RE-TM Alloys: A Review on the Structure and Stability of the γ” Phase and Its Effect on Mechanical Properties.
Metals,
Vol. 13,
Issue. 11,
p.
1856.
Hao, Yiqiang
Chen, Xia
Liu, Zhenyang
and
Chen, Bin
2024.
Transition from γ' to γ'' in Mg-Gd-Zn-Mn alloys investigated by the experiments and combined calculation method (HKD).
Journal of Alloys and Compounds,
Vol. 1005,
Issue. ,
p.
176177.
Xiao, Zhenyu
Xu, Shiwei
Huang, Weiying
Jin, Chen
and
Lin, Zhanhong
2024.
Stability and elastic anisotropy of the β′ and γ'' phases in Mg-Gd-Ag alloys at finite temperatures by first principles calculations.
Journal of Materials Research and Technology,
Vol. 33,
Issue. ,
p.
9195.
Tao, Yao
Chen, Xia
Chen, Qiang
Zheng, Jingxu
and
Chen, Bin
2025.
Atomic-scale investigation and first-principles calculations of γ′′ phase in Mg-Ag-Sm alloys.
Materials Today Communications,
Vol. 46,
Issue. ,
p.
112809.
Tao, Yao
Chen, Xia
Zheng, Jingxu
and
Chen, Bin
2025.
Study on stacking faults in the LaMgAg2 phase of Mg-La-Ag alloy.
Journal of Alloys and Compounds,
Vol. 1021,
Issue. ,
p.
179606.
Li, Chunjuan
Zhao, Sicong
Liu, Kun
Feng, Yicheng
Guo, Erjun
and
Li, Jingfang
2025.
Microstructure and mechanical properties of the Mg-RE-Ag alloys processed by continuous variable channel angular pressing and aging treatment.
Journal of Alloys and Compounds,
Vol. 1032,
Issue. ,
p.
181158.
Yang, Zhenquan
Ma, Aibin
Xu, Bingqian
Wang, Guowei
Jiang, Jinghua
and
Sun, Jiapeng
2025.
Towards age-hardening ability enhancement and high strength in Mg–Gd–Ag alloy by balancing grain refinement and weakening of dynamic precipitation.
Journal of Magnesium and Alloys,
Vol. 13,
Issue. 4,
p.
1699.
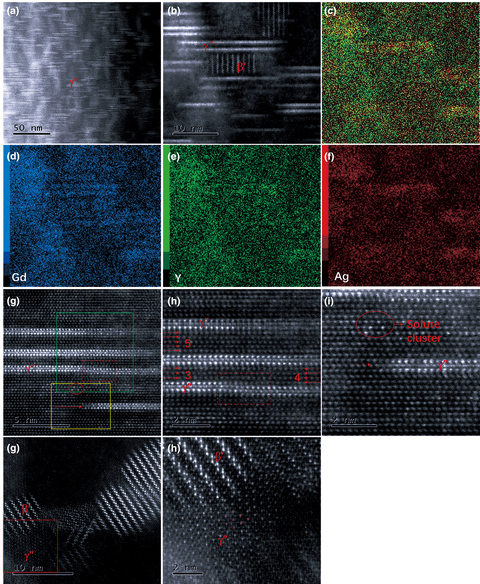
 ${ P\bar{6}}2{ m}$) plays an important role in the strengthening of Mg–Gd–Y–Ag–Zr alloy. In this study, Cs-corrected high-angle annular dark-field scanning transmission electron microscopy was applied to characterize the Mg–Gd–Y–Ag–Zr alloy in different conditions (as-cast, solution-treated, and isothermally aged at 200 °C). The nucleation, growing process, and transformation behavior of the plate-shaped γ″ phase were systematically investigated on the atomic scale. We found that the nucleation sites of the γ″ phase were separated by close-packed planes of the Mg matrix and the γ″ phase developed in two perpendicular directions of
${ P\bar{6}}2{ m}$) plays an important role in the strengthening of Mg–Gd–Y–Ag–Zr alloy. In this study, Cs-corrected high-angle annular dark-field scanning transmission electron microscopy was applied to characterize the Mg–Gd–Y–Ag–Zr alloy in different conditions (as-cast, solution-treated, and isothermally aged at 200 °C). The nucleation, growing process, and transformation behavior of the plate-shaped γ″ phase were systematically investigated on the atomic scale. We found that the nucleation sites of the γ″ phase were separated by close-packed planes of the Mg matrix and the γ″ phase developed in two perpendicular directions of  $\langle 10\bar{1}0 \rangle$ and ⟨0001⟩. The growing process of the γ″ phase on the atomic scale was captured. The γ″ phase was thermodynamically stable at room temperature, and no transformation behavior of the γ″ phase was observed up to 200 h during isothermal aging at 200 °C.
$\langle 10\bar{1}0 \rangle$ and ⟨0001⟩. The growing process of the γ″ phase on the atomic scale was captured. The γ″ phase was thermodynamically stable at room temperature, and no transformation behavior of the γ″ phase was observed up to 200 h during isothermal aging at 200 °C.