Published online by Cambridge University Press: 05 July 2018
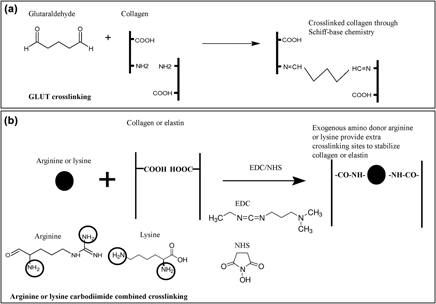
Valvular heart diseases lead to over 300,000 heart valve replacements worldwide each year. Bioprosthetic heart valves (BHVs), derived from glutaraldehyde (GLUT) crosslinked porcine or bovine pericardium, are often used. However, valve failure can occur within 12–15 years due to progressive degradation and/or calcification. Being innovated by previous amino reagent studies used for GLUT detoxification and carbodiimide [1-ethyl-3-(3-dimethylaminopropyl)carbodiimide, EDC] chemistry, in this study, we developed a new fabrication method that utilizes exogenous amino donor arginine or lysine carbodiimide combined treatments to better stabilize the extracellular matrix of porcine pericardium. The carboxyl group density, amine content, differential scanning calorimetry, collagenase and elastase degradation, calcification by rat subdermal implantation, cytotoxicity, and platelet adhesion were characterized. We demonstrated that exogenous amino donor carbodiimide combined treatment for pericardiums had better resistance to elastase degradation (1.63 ± 0.11% and 1.44 ± 0.24% in arginine or lysine versus 3.68 ± 0.16% and 3.04 ± 0.11% in GLUT and GLUT/EDC control) and calcification (0.624 ± 0.193 and 0.637 ± 0.213 Ca µg/mg tissue in arginine or lysine versus 1.610 ± 0.124 and 1.512 ± 0.075 Ca µg/mg tissue in GLUT and GLUT/EDC control). This new strategy combined arginine or lysine and carbodiimide crosslinking would be a promising method to produce more robust BHVs with better structural stability and anticalcification property.