No CrossRef data available.
Published online by Cambridge University Press: 13 February 2017
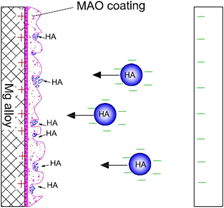
A CeO2/ZrO2-hydroxyapatite (HA) composite bio-ceramic coating was prepared on ZK60 magnesium (Mg) alloy by using micro-arc oxidation (MAO) and electrophoretic deposition (EPD). MAO coating was done as the basal layer was grown in alkaline electrolyte with the addition of nanoparticles (CeO2 and ZrO2) to improve the mechanical properties of coating. A HA coating as the covering layer was deposited on the surface of MAO coating for improving the biological properties of the coating. The phase compositions and morphology of coatings were monitored with X-ray diffraction (XRD) and scanning electron microscopy (SEM), respectively. Adhesion and wear resistance of coatings were evaluated using a scratch test and a pin-on-disc sliding wear test. The corrosion resistance of coatings was evaluated in a simulated body fluid (SBF) using electrochemical tests at 36.5 ± 0.5 °C. The experimental results showed that the CeO2/ZrO2-HA composite coating on Mg alloy effectively improved its mechanical properties and corrosion resistance. Combining MAO and EPD is a promising modification technology for degradable Mg alloys as biomaterials.
Contributing Editor: Jürgen Eckert