Published online by Cambridge University Press: 04 March 2019
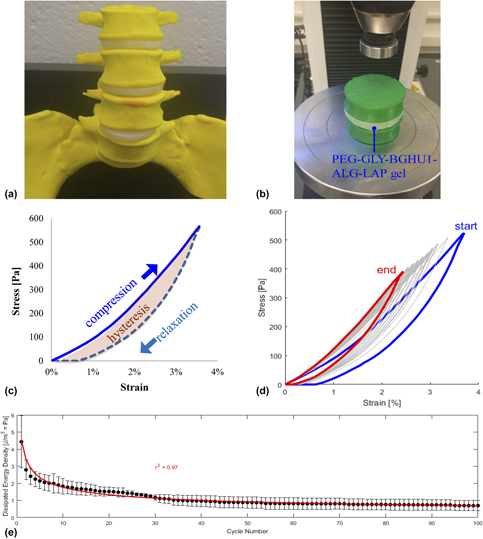
Poly(ethylene glycol) (PEG)-based materials can potentially be used as biomechanical matrices in regenerative medicine and tissue engineering implants including the replacement of intervertebral (IV) disks. Glycerol and other generally recognized as safe (GRAS) plasticizers (low-MW PEG, propylene glycol, and sorbitol) were added to the bulk PEG matrix and gelled using chemical and photochemical methods at different temperatures (21, 37, 59, and 80 °C) and pressures (0 and 90 MPa gauge) settings, and their compression testing properties were acquired and analyzed. Surface incorporation of custom-made bioactive glass particles shortened the blood clotting time (78% compared to no glass particles), while alginate and laponite additives improved the gel’s mechanical properties to 645 kPa compressive modulus, 12% yield strain, and 79 kPa yield strength. This IV disk-modeled hydrogel system endured the cyclic loading and unloading tests at 4% compressive strain indicative of an elastic response, but required improvement to its biomechanical tolerance for downstream bioengineering applications.