Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by
Crossref.
Kan, Akinori
Moriyama, Tohru
Takahashi, Susumu
and
Ogawa, Hirotaka
2012.
Microwave Dielectric Properties of LiF- and CaTiO3-Added MgO with Low Dielectric Loss and Near-Zero Temperature Coefficient of Resonant Frequency for Low-Temperature Cofired-Ceramic.
Japanese Journal of Applied Physics,
Vol. 51,
Issue. 9S1,
p.
09LF01.
Zhou, Juehui
Yang, Hui
Xu, Nuoxin
and
Zhang, Qilong
2014.
Enhanced effect of non-stoichiometry on the sinterability and microwave dielectric properties of Mg2+x SnO4 ceramics.
Journal of Materials Science: Materials in Electronics,
Vol. 25,
Issue. 1,
p.
500.
Kim, Shin
Kim, So-Jung
Nam, Kyung-Jin
Cha, Hansol
and
Yoon, Sang-Ok
2015.
Thermal and Dielectric Properties of LiF-Doped MgO Ceramics.
Journal of the Korean Institute of Electrical and Electronic Material Engineers,
Vol. 28,
Issue. 7,
p.
419.
Wang, Min-Jia
Yang, Hui
Zhang, Qi-Long
Yu, Dan
Hu, Liang
Lin, Zhi-Sheng
and
Zhang, Zi-Shan
2015.
Low temperature sintering properties of LiF-doped BaTiO3-based dielectric ceramics for AC MLCCs.
Journal of Materials Science: Materials in Electronics,
Vol. 26,
Issue. 1,
p.
162.
Kusne, A G
Keller, D
Anderson, A
Zaban, A
and
Takeuchi, I
2015.
High-throughput determination of structural phase diagram and constituent phases using GRENDEL.
Nanotechnology,
Vol. 26,
Issue. 44,
p.
444002.
Chen, Miaoqin
He, Jinjiang
Zhang, Yuli
Ding, Zhaochong
and
Luo, Junfeng
2017.
Densification and grain growth behaviour of high-purity MgO ceramics by hot-pressing.
Ceramics International,
Vol. 43,
Issue. 2,
p.
1775.
Yang, C.H.
Liu, Q.Q.
Bi, J.X.
Tao, W.H.
and
Wu, H.T.
2018.
Effects of (Mg1/3Ta2/3)-substitution for Ti-site on the microwave dielectric properties of Li2MgTiO4 ceramics.
Ceramics International,
Vol. 44,
Issue. 6,
p.
5982.
Yang, C.H.
and
Wu, H.T.
2018.
Phase composition, Raman spectra, infrared spectra and dielectric properties of Li2MgTi1-x(Mg1/3Nb2/3)xO4 ceramics at microwave frequency.
Ceramics International,
Vol. 44,
Issue. 8,
p.
9255.
Lai, Yuanming
Su, Hua
Wang, Gang
Tang, Xiaoli
Huang, Xin
Liang, Xiaofeng
Zhang, Huaiwu
Li, Yuanxun
Huang, Ke
and
Wang, Xiao Renshaw
2019.
Low‐temperature sintering of microwave ceramics with high Qf values through LiF addition.
Journal of the American Ceramic Society,
Vol. 102,
Issue. 4,
p.
1893.
Lu, Nan
He, Gang
Yang, Zengchao
Li, Yong
and
Li, Jiangtao
2020.
Fabrication and densification mechanism of MgO/Graphene composites with LiF as additive.
Scripta Materialia,
Vol. 174,
Issue. ,
p.
91.
Tan, Zhenyu
Lin, Huixing
Song, Kaixin
Dang, Mingzhao
Yao, Xiaogang
Ren, Haishen
Xie, Tianyi
and
Peng, Haiyi
2020.
Effects of TiO2 additive on ultra-low-loss MgO–LiF microwave dielectric ceramics.
Ceramics International,
Vol. 46,
Issue. 5,
p.
5753.
Zhu, Shengkai
Huang, Zhichao
Lou, Weichao
Song, Kaixin
Khesro, Amir
Hussain, Fayaz
Tan, Zhenyu
Luo, Xinjiang
Mao, Minmin
Xue, Lingyun
Xu, Ping
Liu, Bing
Lin, Huixing
and
Wang, Dawei
2021.
5G microstrip patch antenna and microwave dielectric properties of 4 mol%LiF–MgO–xwt%MTiO3 (M = Ca, Sr) composite ceramics.
Journal of Materials Science: Materials in Electronics,
Vol. 32,
Issue. 19,
p.
23880.
Zhang, Chi
Chen, Ying
Li, Xin
Ren, Haishen
Wang, Genshui
and
Dong, Xianlin
2021.
Effect of LiF addition on sintering behavior and dielectric breakdown mechanism of MgO-based microwave dielectric ceramics.
Journal of Materiomics,
Vol. 7,
Issue. 3,
p.
478.
Zhang, Chi
Huang, Jian
Chen, Ying
Cai, Ziming
Wang, Genshui
and
Dong, Xianlin
2021.
Combined Merits of High Dielectric Breakdown Strength and Low Sintering Temperature Acquired in Mgo-Baseddielectric Ceramics: From Theoretical Prediction to Experimental Validation.
SSRN Electronic Journal ,
Zhang, Chi
Huang, Jian
Chen, Ying
Cai, Ziming
Wang, Genshui
and
Dong, Xianlin
2022.
Combined merits of high dielectric breakdown strength and low sintering temperature acquired in MgO-based dielectric ceramics: from theoretical prediction to experimental validation.
Acta Materialia,
Vol. 226,
Issue. ,
p.
117610.
Zhang, Hao-jie
Li, Qian
Gu, Yong-jun
Li, Li-hua
Huang, Jin-liang
and
Kim, Bok-hee
2023.
Effect of LiF on microwave dielectric properties of nonstoichiometric Mg2SiO4 derived using deep eutectic solvents.
Journal of Materials Science: Materials in Electronics,
Vol. 34,
Issue. 9,
Kan, Akinori
Hirabayashi, Ryosuke
Takahashi, Susumu
and
Ogawa, Hirotaka
2023.
Low-temperature crystallization and microwave dielectric properties of forsterite generated in MgO–SiO2 system following LiF addition.
Ceramics International,
Vol. 49,
Issue. 6,
p.
9883.
Shi, Zitao
Fang, Zixuan
Zhang, Shuren
and
Tang, Bin
2024.
Cracking behavior of Sm2O3 ceramics and enhanced microwave dielectric properties with MgO addition: Insights from chemical bond characteristics and microstructure.
Ceramics International,
Vol. 50,
Issue. 3,
p.
5434.
Halabi, Rawan
Simotko, Sasha
and
Tsur, Yoed
2024.
The influence of point defects on the sintering of magnesium oxide.
Journal of the American Ceramic Society,
Vol. 107,
Issue. 12,
p.
8023.
Bian, J. J.
and
Liu, Q. R.
2025.
Effects of Li+ and/or F− modification methods on the sintering behaviors, microstructures of Mg2SiO4 ceramics.
International Journal of Applied Ceramic Technology,
Vol. 22,
Issue. 3,
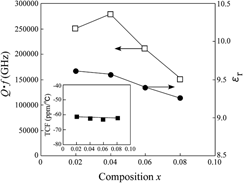
 values strongly depended on the LiF content. As a result, the highest
values strongly depended on the LiF content. As a result, the highest  value of 282,230 GHz was obtained at x = 0.04. From these results, it is determined that the LiF addition is effective in reducing the sintering temperature of MgO without any detrimental effect on the microwave dielectric properties of MgO ceramics.
value of 282,230 GHz was obtained at x = 0.04. From these results, it is determined that the LiF addition is effective in reducing the sintering temperature of MgO without any detrimental effect on the microwave dielectric properties of MgO ceramics.
