No CrossRef data available.
Published online by Cambridge University Press: 28 May 2019
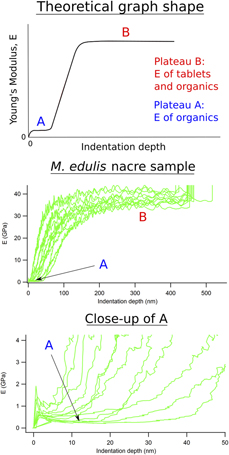
A new protocol has been devised for determining elastic properties of natural biocomposites in the form of bivalve shells under wet and dry conditions. Four-point bending on shell slices of Mytilus edulis, Ensis siliqua, and Pecten maximus give generally lower and more reliable values of Young’s modulus, E, than those in the literature from three-point bending, due to the more even distribution of strain. Finite element analysis of the prismatic microstructure of Pinna nobilis, obtained by X-ray tomography, shows that values of E ≈ 20 GPa can be understood in terms of the real microstructure containing a small proportion of organic matrix phase with E ≈ 1 GPa and a dominant proportion of calcite with E ≈ 90 GPa. Higher values of E obtained by nanoindentation give results which are biased toward the properties of the carbonate phase rather than of the biocomposite as a whole.