Published online by Cambridge University Press: 26 May 2020
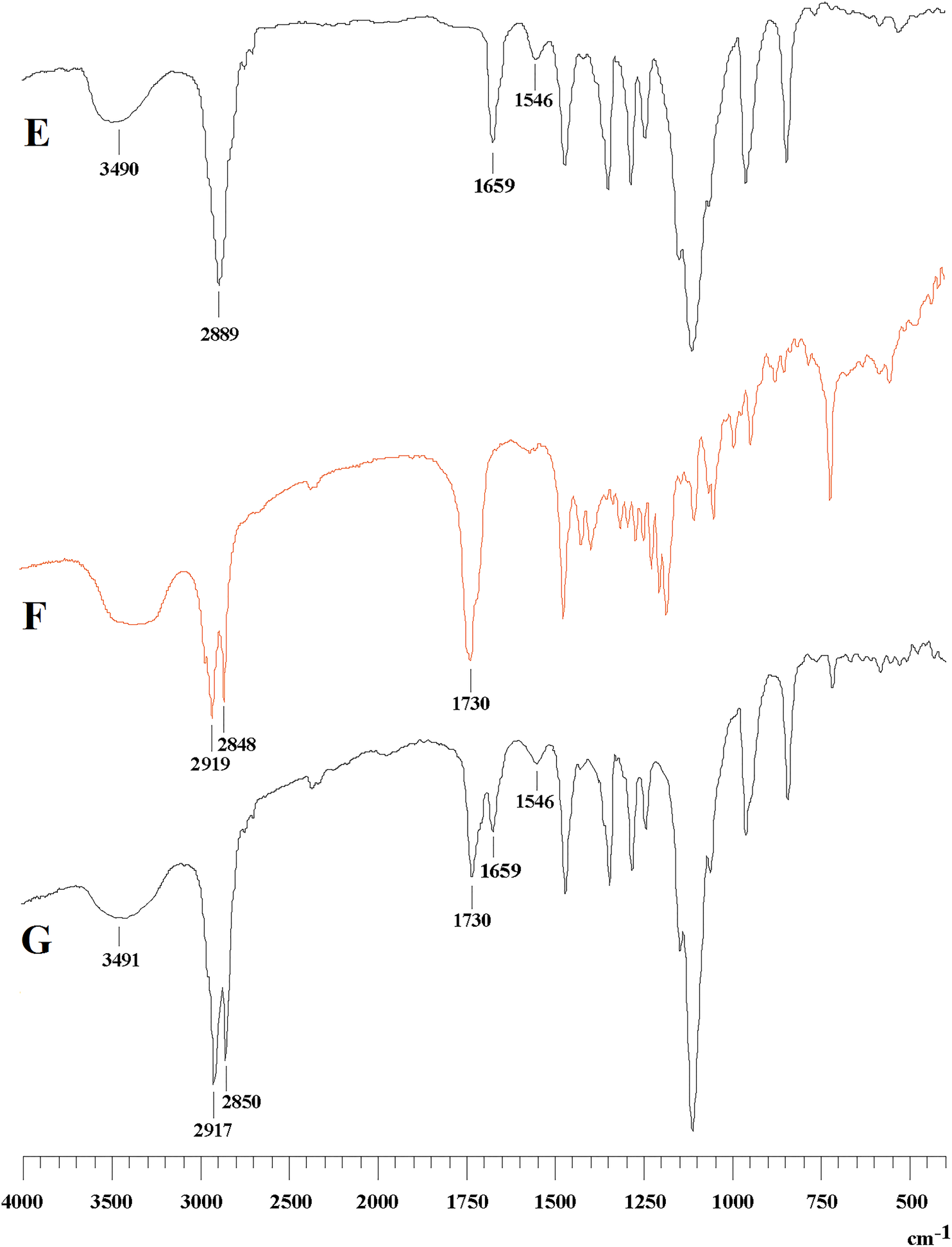
The purpose of this study was to construct a glycyrrhetinic acid (GA)-mediated, breakable, intracellular, nanoscale drug-delivery carrier via amide and esterification reactions. The structures were identified by Fourier-transformed infrared (FTIR) and 1H-nuclear magnetic resonance (1H-NMR) spectrophotometry. The compatibility and safety of the carrier were evaluated using hemolysis and cytotoxicity tests. The GA-copolymer micelle was prepared using the solvent evaporation method. FTIR and 1H-NMR detection demonstrated the successful construction of the polymer. No hemolysis occurred in any concentration of polymer within 3 h, and the hemolysis rate was less than 5%. 3-(4,5-dimethyl-thiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) experimental results showed that the novel polymer reduced the cell survival rate and had significant cytotoxic effects. The blank nanoparticles were liquid with light blue opalescence. Transmission electron microscopy revealed that the empty micelles were uniform spheres, with an average size of 62 nm and a zeta potential of −13 mV. The novel GA-mediated polymeric carrier material developed here has the potential to effectively kill human SMMC-7721 cancer cells within 3 days when the dose is above 500 ug/mL.