Published online by Cambridge University Press: 16 May 2017
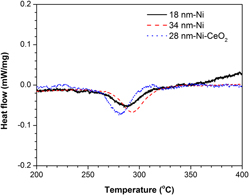
Thermal stability up to 400 °C of nanocrystalline (NC) Ni electrodeposits (EDs) with a mean grain size of 28 nm and dispersions of a small amount of CeO2 nanoparticles has been investigated by comparing with two CeO2-free NC Ni counterparts, one with a slightly smaller mean grain size of 18 nm and the other with a slightly larger mean grain size of 34 nm. The results show that the co-deposition of CeO2 particles has dual effects on the thermal stability of the NC Ni EDs, i.e., it promotes the grain growth at the beginning but retards subsequently. It is proposed that the CeO2 co-deposition leads to a decrease in sulfur level and an increase in the plane defects as a result of introduction of incoherent Ni/CeO2 interfaces, which play dominant roles in the grain growth at low temperatures; while the drag effect of CeO2 dispersions becomes dominant at higher temperatures.
Contributing Editor: Susan B. Sinnott